Bioaccessibility and Bioavailability of Minerals in Relation to a Healthy Gut Microbiome
- PMID: 34202712
- PMCID: PMC8268569
- DOI: 10.3390/ijms22136803
Bioaccessibility and Bioavailability of Minerals in Relation to a Healthy Gut Microbiome
Abstract
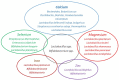
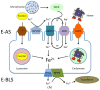
Adequate amounts of a wide range of micronutrients are needed by body tissues to maintain health. Dietary intake must be sufficient to meet these micronutrient requirements. Mineral deficiency does not seem to be the result of a physically active life or of athletic training but is more likely to arise from disturbances in the quality and quantity of ingested food. The lack of some minerals in the body appears to be symbolic of the modern era reflecting either the excessive intake of empty calories or a negative energy balance from drastic weight-loss diets. Several animal studies provide convincing evidence for an association between dietary micronutrient availability and microbial composition in the gut. However, the influence of human gut microbiota on the bioaccessibility and bioavailability of trace elements in human food has rarely been studied. Bacteria play a role by effecting mineral bioavailability and bioaccessibility, which are further increased through the fermentation of cereals and the soaking and germination of crops. Moreover, probiotics have a positive effect on iron, calcium, selenium, and zinc in relation to gut microbiome composition and metabolism. The current literature reveals the beneficial effects of bacteria on mineral bioaccessibility and bioavailability in supporting both the human gut microbiome and overall health. This review focuses on interactions between the gut microbiota and several minerals in sport nutrition, as related to a physically active lifestyle.
Keywords: Fe deficiency; gut microbiota; magnesium; micronutrient; physical fitness; trace element.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Rodriguez N.R., Di Marco N.M., Langley S. American College of Sports Medicine position stand. Nutrition and athletic performance. Med. Sci. Sports Exerc. 2009;41:709–731. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

