Optimization of Moist and Oven-Dried Bacterial Cellulose Production for Functional Properties
- PMID: 34202870
- PMCID: PMC8272063
- DOI: 10.3390/polym13132088
Optimization of Moist and Oven-Dried Bacterial Cellulose Production for Functional Properties
Erratum in
-
Correction: Bodea et al. Optimization of Moist and Oven-Dried Bacterial Cellulose Production for Functional Properties. Polymers 2021, 13, 2088.Polymers (Basel). 2021 Nov 5;13(21):3821. doi: 10.3390/polym13213821. Polymers (Basel). 2021. PMID: 34771415 Free PMC article.
Abstract
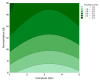
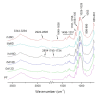
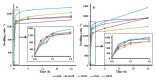
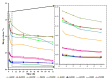
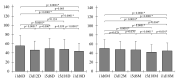
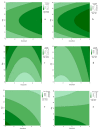
Bacterial cellulose (BC) is a natural polymer with properties suitable for tissue engineering and possible applications in scaffold production. However, current procedures have limitations in obtaining BC pellicles with the desired structural, physical, and mechanical properties. Thus, this study analyzed the optimal culture conditions of BC membranes and two types of processing: draining and oven-drying. The aim was to obtain BC membranes with properties suitable for a wound dressing material. Two studies were carried out. In the preliminary study, the medium (100 mL) was inoculated with varying volumes (1, 2, 3, 4, and 5 mL) and incubated statically for different periods (3, 6, 9, 12, and 18 days), using a full factorial experimental design. Thickness, uniformity, weight, and yield were evaluated. In the optimization study, a Box-Behnken design was used. Two independent variables were used: inoculum volume (X1: 1, 3, and 5 mL) and fermentation period (X2: 6, 12, and 18 d) to determine the target response variables: thickness, swelling ratio, drug release, fiber diameter, tensile strength, and Young's modulus for both dry and moist BC membranes. The mathematical modelling of the effect of the two independent variables was performed by response surface methodology (RSM). The obtained models were validated with new experimental values and confirmed for all tested properties, except Young's modulus of oven-dried BC. Thus, the optimal properties in terms of a scaffold material of the moist BC were obtained with an inoculum volume of 5% (v/v) and 16 d of fermentation. While, for the oven-dried membranes, optimal properties were obtained with a 4% (v/v) and 14 d of fermentation.
Keywords: SEM; TEM; drug-release; swelling ratio; thickness.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Kalkhoran A.H.Z., Naghib S.M., Vahidi O., Rahmanian M. Synthesis and characterization of graphene-grafted gelatin nanocomposite hydrogels as emerging drug delivery systems. Biomed. Phys. Eng. Express. 2018;4:055017. doi: 10.1088/2057-1976/aad745. - DOI
-
- Ullah H., Badshah M., Mäkilä E., Salonen J., Shahbazi M.-A., Santos H.A., Khan T. Fabrication, characterization and evaluation of bacterial cellulose-based capsule shells for oral drug delivery. Cellulose. 2017;24:1445–1454. doi: 10.1007/s10570-017-1202-4. - DOI
-
- Badshah M., Ullah H., Khan S.A., Park J.K., Khan T. Preparation, characterization and in-vitro evaluation of bacterial cellulose matrices for oral drug delivery. Cellulose. 2017;24:5041–5052. doi: 10.1007/s10570-017-1474-8. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

