RSK Isoforms in Acute Myeloid Leukemia
- PMID: 34202904
- PMCID: PMC8301392
- DOI: 10.3390/biomedicines9070726
RSK Isoforms in Acute Myeloid Leukemia
Abstract
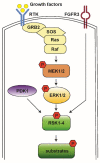
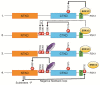
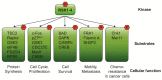
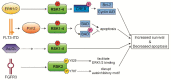
Ribosomal S6 Kinases (RSKs) are a group of serine/threonine kinases that function downstream of the Ras/Raf/MEK/ERK signaling pathway. Four RSK isoforms are directly activated by ERK1/2 in response to extracellular stimuli including growth factors, hormones, and chemokines. RSKs phosphorylate many cytosolic and nuclear targets resulting in the regulation of diverse cellular processes such as cell proliferation, survival, and motility. In hematological malignancies such as acute myeloid leukemia (AML), RSK isoforms are highly expressed and aberrantly activated resulting in poor outcomes and resistance to chemotherapy. Therefore, understanding RSK function in leukemia could lead to promising therapeutic strategies. This review summarizes the current information on human RSK isoforms and discusses their potential roles in the pathogenesis of AML and mechanism of pharmacological inhibitors.
Keywords: AML; RSK inhibitors; RSK isoforms; cancer; hematological malignancy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

