Alleviation of Memory Deficit by Bergenin via the Regulation of Reelin and Nrf-2/NF-κB Pathway in Transgenic Mouse Model
- PMID: 34203049
- PMCID: PMC8234641
- DOI: 10.3390/ijms22126603
Alleviation of Memory Deficit by Bergenin via the Regulation of Reelin and Nrf-2/NF-κB Pathway in Transgenic Mouse Model
Abstract
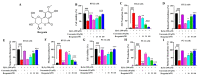
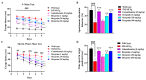
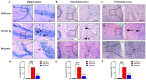
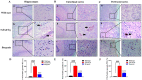
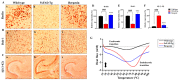
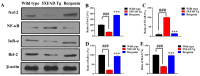
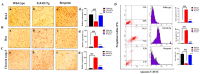
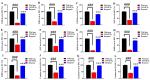
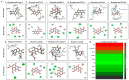
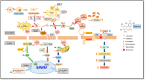
The present study aims to determine the neuroprotective effect of Bergenin against spatial memory deficit associated with neurodegeneration. Preliminarily, the protective effect of Bergenin was observed against H2O2-induced oxidative stress in HT-22 and PC-12 cells. Further studies were performed in 5xFAD Tg mouse model by administering Bergenin (1, 30 and 60 mg/kg; orally), whereas Bergenin (60 mg/kg) significantly attenuated the memory deficit observed in the Y-maze and Morris water maze (MWM) test. Fourier transform-infrared (FT-IR) spectroscopy displayed restoration of lipids, proteins and their derivatives compared to the 5xFAD Tg mice group. The differential scanning calorimeter (DSC) suggested an absence of amyloid beta (Aβ) aggregation in Bergenin-treated mice. The immunohistochemistry (IHC) analysis suggested the neuroprotective effect of Bergenin by increasing Reelin signaling (Reelin/Dab-1) and attenuated Aβ (1-42) aggregation in hippocampal regions of mouse brains. Furthermore, IHC and western blot results suggested antioxidant (Keap-1/Nrf-2/HO-1), anti-inflammatory (TLR-4/NF-kB) and anti-apoptotic (Bcl-2/Bax/Caspase-3) effect of Bergenin. Moreover, a decrease in Annexin V/PI-stained hippocampal cells suggested its effect against neurodegeneration. The histopathological changes were reversed significantly by Bergenin. In addition, a remarkable increase in antioxidant level with suppression of pro-inflammatory cytokines, oxidative stress and nitric oxide production were observed in specific regions of the mouse brains.
Keywords: Alzheimer’s disease; Bergenin; Reelin signaling; memory loss; neurodegeneration; oxidative stress.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Jawhar S., Trawicka A., Jenneckens C., Bayer T.A., Wirths O. Motor deficits, neuron loss, and reduced anxiety coinciding with axonal degeneration and intraneuronal Aβ aggregation in the 5XFAD mouse model of Alzheimer’s disease. Neurobiol. Aging. 2012;33:196.e29–196.e40. doi: 10.1016/j.neurobiolaging.2010.05.027. - DOI - PubMed
-
- Albert M.S., DeKosky S.T., Dickson D., Dubois B., Feldman H.H., Fox N.C., Gamst A., Holtzman D.M., Jagust W.J., Petersen R.C., et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008. - DOI - PMC - PubMed
-
- Chin J., Massaro C.M., Palop J.J., Thwin M.T., Yu G.Q., Bien-Ly N., Bender A., Mucke L. Reelin depletion in the entorhinal cortex of human amyloid precursor protein transgenic mice and humans with Alzheimer’s disease. J. Neurosci. Off. J. Soc. Neurosci. 2007;27:2727–2733. doi: 10.1523/JNEUROSCI.3758-06.2007. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

