The Viral Capsid: A Master Key to Access the Host Nucleus
- PMID: 34203080
- PMCID: PMC8234750
- DOI: 10.3390/v13061178
The Viral Capsid: A Master Key to Access the Host Nucleus
Abstract
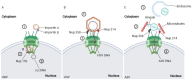
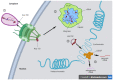
Viruses are pathogens that have evolved to hijack the cellular machinery to replicate themselves and spread to new cells. During the course of evolution, viruses developed different strategies to overcome the cellular defenses and create new progeny. Among them, some RNA and many DNA viruses require access to the nucleus to replicate their genome. In non-dividing cells, viruses can only access the nucleus through the nuclear pore complex (NPC). Therefore, viruses have developed strategies to usurp the nuclear transport machinery and gain access to the nucleus. The majority of these viruses use the capsid to manipulate the nuclear import machinery. However, the particular tactics employed by each virus to reach the host chromatin compartment are very different. Nevertheless, they all require some degree of capsid remodeling. Recent notions on the interplay between the viral capsid and cellular factors shine new light on the quest for the nuclear entry step and for the fate of these viruses. In this review, we describe the main components and function of nuclear transport machinery. Next, we discuss selected examples of RNA and DNA viruses (HBV, HSV, adenovirus, and HIV) that remodel their capsid as part of their strategies to access the nucleus and to replicate.
Keywords: host–viral interactions; viral nuclear entry; viral replication.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Löschberger A., Linde S., van de Dabauvalle M.-C., Rieger B., Heilemann M., Krohne G., Sauer M. Super-resolution imaging visualizes the eightfold symmetry of gp210 proteins around the nuclear pore complex and resolves the central channel with nanometer resolution. J. Cell Sci. 2012;125:570–575. doi: 10.1242/jcs.098822. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

