Randomized Controlled Trial of Simple Salt Reduction Instructions by Physician for Patients with Type 2 Diabetes Consuming Excessive Salt
- PMID: 34203155
- PMCID: PMC8297346
- DOI: 10.3390/ijerph18136913
Randomized Controlled Trial of Simple Salt Reduction Instructions by Physician for Patients with Type 2 Diabetes Consuming Excessive Salt
Abstract
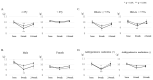
Objectives: We verified the clinical usefulness of an approach method in which a physician gives simple salt reduction instructions during outpatient visits to patients with type 2 diabetes. Methods: This study was an open-blind, randomized controlled trial. Subjects were outpatients with type 2 diabetes whose estimated salt intake using spot morning urine sample exceeded the target of salt intake. The control group (CG) was notified only of the current salt intake, whereas the intervention group (IG) was given the brief salt reduction instruction by a physician in addition to the information regarding their current salt intake. Results: The change in estimated salt intake was -0.6 g (from 10.1 to 9.5 g, p = 0.029) in the CG after 8 weeks, and -0.9 g (from 10.1 to 9.2 g, p = 0.001) in the IG, although there were no significant differences between them (p = 0.47). After 24 weeks, both groups no longer differed significantly from the baseline. In addition, multivariate linear regression analyses indicated that high salt intake and low estimated glomerular filtration rate at baseline were significantly associated with salt reduction after 8 weeks. Conclusions: Salt-reducing effects were observed after 8 weeks in both the IG and CG, but no significant difference was observed. Moreover, patients with high salt intake and renal disfunction may be more effective in accepting salt reduction instructions. Making patients aware of the importance of salt reduction through a physician is effective for continuous salt reduction, and it is important to continue regular and repetitive guidance.
Keywords: hypertension; nutritional instructions; randomized controlled trial; salt intake; salt restriction; type 2 diabetes mellitus.
Conflict of interest statement
Chikako Oyabu has received personal fees from Sanofi K.K., Kowa Pharmaceutical Co., Ltd., Novo Nordisk Pharma Ltd., Ono Pharma Co., Ltd., Taisho Pharma Co., Ltd., AstraZeneca K.K., outside the submitted work. Emi Ushigome has received grants from the Astellas Foundation for Research on Metabolic Disorders (Grant number: 4024), the Japanese Study Group for Physiology and Management of Blood Pressure. Donated Fund Laboratory of Diabetes therapeutics is an endowment department, supported with an unrestricted grant from Ono Pharmaceutical Co., Ltd., and received personal fees from Astellas Pharma Inc., AstraZeneca plc, Daiichi Sankyo Co., Ltd., Kowa Pharmaceutical Co., Ltd., Kyowa Hakko Kirin Company Ltd., MSD K.K., Novo Nordisk Pharma Ltd., Mitsubishi Tanabe Pharma Corp., Taisho Toyama Pharmaceutical Co., Ltd., Nippon Boehringer Ingelheim Co., Takeda Pharmaceutical Co., Ltd., Ltd., and Sumitomo Dainippon Pharma Co., Ltd., outside the submitted work. Ayaka Kobayashi has received personal fees from Mitsubishi Tanabe Pharma Co, Sanofi K.K., MSD K.K., Ltd., Novo Nordisk Pharma Ltd., Eli Lilly Japan K.K., outside the submitted work. Yoshitaka Hashimoto has received grants from Asahi Kasei Pharma, personal fees from Mitsubishi Tanabe Pharma Corp., Daiichi Sankyo Co., Ltd., Sanofi K.K., Novo Nordisk Pharma Ltd., outside the submitted work. Ryosuke Sakai has received personal fees from Mitsubishi Tanabe Pharma Co, Daiichi Sankyo Co., Ltd., Sumitomo Dainippon Pharma Co., Ltd., Kyowa Hakko Kirin Co., MSD K.K., outside the submitted work. Hiroya Iwase has received personal fees from Nippon Boehringer Ingelheim Co., Ltd., Mitsubishi Tanabe Pharma Co, Daiichi Sankyo Co., Ltd., Sanofi K.K., Astellas Pharma Inc., Sumitomo Dainippon Pharma Co., Ltd., Kyowa Hakko Kirin Co., Kowa Pharmaceutical Co., Ltd., Novo Nordisk Pharma Ltd., Ono Pharma Co., Ltd., Eli Lilly Japan K.K., Taisho Pharma Co., Ltd., Abbott Japan Co., Ltd., AstraZeneca K.K., Medtronic Japan Co., Ltd., outside the submitted work. Hiroshi Okada has received personal fees from MSD K.K., Sumitomo Dainippon Pharma Co., Ltd., Novo Nordisk Pharma Ltd., Daiichi Sankyo Co., Ltd., Eli Lilly Japan K.K, Takeda Pharmaceutical Co., Ltd., Terumo Co., Bayer Yakuhin, Ltd., outside the submitted work. Michiaki Fukui has received grants from Nippon Boehringer Ingelheim Co., Ltd., Mitsubishi Tanabe Pharma Co, Kissei Pharma Co., Ltd., Daiichi Sankyo Co., Ltd., Takeda Pharma Co., Ltd., Sanofi K.K., Astellas Pharma Inc., MSD K.K., Ltd., Sumitomo Dainippon Pharma Co., Ltd., Kyowa Hakko Kirin Co., Kowa Pharmaceutical Co., Ltd., Sanwa Kagaku Kenkyusho Co., Ltd. Novo Nordisk Pharma Ltd., Ono Pharma Co., Ltd., Eli Lilly Japan K.K., Taisho Pharma Co., Ltd., Terumo Co., Abbott Japan Co., Ltd., Teijin Pharma Ltd., Nippon Chemiphar Co., Ltd., Johnson & Johnson K.K. Medical Co., and received personal fees from Kissei Pharma Co., Nippon Boehringer Ingelheim Co., Ltd., Ltd., Mitsubishi Tanabe Pharma Corp., Sanofi K.K., Takeda Pharma Co., Ltd., Daiichi Sankyo Co., Ltd., Astellas Pharma Inc., MSD K.K., Kyowa Kirin Co., Ltd., Kowa Pharma Co., Ltd., Novo Nordisk Pharma Ltd., Sumitomo Dainippon Pharma Co., Ltd., Ono Pharma Co., Ltd., Eli Lilly Japan K.K., Taisho Pharma Co., Ltd., Sanwa Kagaku Kenkyusho Co., Ltd., Bayer Yakuhin, Ltd., AstraZeneca K.K., Abbott Japan Co., Ltd., Medtronic Japan Co., Ltd., Mochida Pharma Co., Ltd., Arkley Inc., Teijin Pharma Ltd. and Nipro Cor., outside the submitted work. Toru Tanaka has received personal fees from Nippon Boehringer Ingelheim Co., Ltd., Mitsubishi Tanabe Pharma Co, Kissei Pharma Co., Ltd., Daiichi Sankyo Co., Ltd., Takeda Pharma Co., Ltd., Sanofi K.K., Astellas Pharma Inc., MSD K.K., Ltd., Sumitomo Dainippon Pharma Co., Ltd., Kyowa Hakko Kirin Co., Kowa Pharmaceutical Co., Ltd., Sanwa Kagaku Kenkyusho Co., Ltd. Novo Nordisk Pharma Ltd., Ono Pharma Co., Ltd., Eli Lilly Japan K.K., Taisho Pharma Co., Ltd., Terumo Co., Teijin Pharma Ltd., Johnson & Johnson K.K. Medical Co., AstraZeneca K.K., outside the submitted work.
Figures

References
-
- Horikawa C., Yoshimura Y., Kamada C., Tanaka S., Tanaka S., Hanyu O., Araki A., Ito H., Tanaka A., Ohashi Y., et al. Dietary sodium intake and incidence of diabetes complications in Japanese patients with type 2 diabetes: Analysis of the Japan diabetes complications study (JDCS) J. Clin. Endocrinol. Metab. 2014 doi: 10.1210/jc.2013-4315. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

