Disruption of O-Linked N-Acetylglucosamine Signaling in Placenta Induces Insulin Sensitivity in Female Offspring
- PMID: 34203166
- PMCID: PMC8267851
- DOI: 10.3390/ijms22136918
Disruption of O-Linked N-Acetylglucosamine Signaling in Placenta Induces Insulin Sensitivity in Female Offspring
Abstract
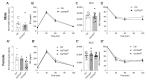
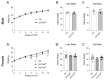
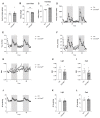
Placental dysfunction can lead to fetal growth restriction which is associated with perinatal morbidity and mortality. Fetal growth restriction increases the risk of obesity and diabetes later in life. Placental O-GlcNAc transferase (OGT) has been identified as a marker and a mediator of placental insufficiency in the setting of prenatal stress, however, its role in the fetal programming of metabolism and glucose homeostasis remains unknown. We aim to determine the long-term metabolic outcomes of offspring with a reduction in placental OGT. Mice with a partial reduction and a full knockout of placenta-specific OGT were generated utilizing the Cre-Lox system. Glucose homeostasis and metabolic parameters were assessed on a normal chow and a high-fat diet in both male and female adult offspring. A reduction in placental OGT did not demonstrate differences in the metabolic parameters or glucose homeostasis compared to the controls on a standard chow. The high-fat diet provided a metabolic challenge that revealed a decrease in body weight gain (p = 0.02) and an improved insulin tolerance (p = 0.03) for offspring with a partially reduced placental OGT but not when OGT was fully knocked out. Changes in body weight were not associated with changes in energy homeostasis. Offspring with a partial reduction in placental OGT demonstrated increased hepatic Akt phosphorylation in response to insulin treatment (p = 0.02). A partial reduction in placental OGT was protective from weight gain and insulin intolerance when faced with the metabolic challenge of a high-fat diet. This appears to be, in part, due to increased hepatic insulin signaling. The findings of this study contribute to the greater understanding of fetal metabolic programming and the effect of placental OGT on peripheral insulin sensitivity and provides a target for future investigation and clinical applications.
Keywords: O-GlcNAcylation; O-linked N-acetylglucosamine (GlcNAc) transferase; OGT; beta-cells; fetal programming; glucose homeostasis; islet; metabolism; pancreas; placenta.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

