Evolution of Terpene Synthases in Orchidaceae
- PMID: 34203299
- PMCID: PMC8268431
- DOI: 10.3390/ijms22136947
Evolution of Terpene Synthases in Orchidaceae
Abstract
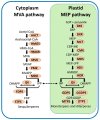
Terpenoids are the largest class of plant secondary metabolites and are one of the major emitted volatile compounds released to the atmosphere. They have functions of attracting pollinators or defense function, insecticidal properties, and are even used as pharmaceutical agents. Because of the importance of terpenoids, an increasing number of plants are required to investigate the function and evolution of terpene synthases (TPSs) that are the key enzymes in terpenoids biosynthesis. Orchidacea, containing more than 800 genera and 28,000 species, is one of the largest and most diverse families of flowering plants, and is widely distributed. Here, the diversification of the TPSs evolution in Orchidaceae is revealed. A characterization and phylogeny of TPSs from four different species with whole genome sequences is available. Phylogenetic analysis of orchid TPSs indicates these genes are divided into TPS-a, -b, -e/f, and g subfamilies, and their duplicated copies are increased in derived orchid species compared to that in the early divergence orchid, A. shenzhenica. The large increase of both TPS-a and TPS-b copies can probably be attributed to the pro-duction of different volatile compounds for attracting pollinators or generating chemical defenses in derived orchid lineages; while the duplications of TPS-g and TPS-e/f copies occurred in a species-dependent manner.
Keywords: Orchidaceae; evolution; phylogenetic tree; terpene synthase.
Conflict of interest statement
No conflict of interest declared.
Figures





References
-
- El Tamer M.K., Lücker J., Bosch D., Verhoeven H.A., Verstappen F.W., Schwab W., van Tunen A.J., Voragen A.G., De Maagd R.A., Bouwmeester H.J. Domain swapping of Citrus limon monoterpene synthases: Impact on enzymatic activity and product specificity. Arch. Biochem. Biophys. 2003;411:196–203. doi: 10.1016/S0003-9861(02)00711-7. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

