Pathogenesis of Anorectal Malformations in Retinoic Acid Receptor Knockout Mice Studied by HREM
- PMID: 34203310
- PMCID: PMC8301324
- DOI: 10.3390/biomedicines9070742
Pathogenesis of Anorectal Malformations in Retinoic Acid Receptor Knockout Mice Studied by HREM
Abstract
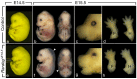
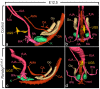
Anorectal malformations (ARMs) are relatively common congenital abnormalities, but their pathogenesis is poorly understood. Previous gene knockout studies indicated that the signalling pathway mediated by the retinoic acid receptors (RAR) is instrumental to the formation of the anorectal canal and of various urogenital structures. Here, we show that simultaneous ablation of the three RARs in the mouse embryo results in a spectrum of malformations of the pelvic organs in which anorectal and urinary bladder ageneses are consistently associated. We found that these ageneses could be accounted for by defects in the processes of growth and migration of the cloaca, the embryonic structure from which the anorectal canal and urinary bladder originate. We further show that these defects are preceded by a failure of the lateral shift of the umbilical arteries and propose vascular abnormalities as a possible cause of ARM. Through the comparisons of these phenotypes with those of other mutant mice and of human patients, we would like to suggest that morphological data may provide a solid base to test molecular as well as clinical hypotheses.
Keywords: Fraser syndrome; congenital malformations; mesenteric artery; mouse embryonic development; umbilical artery; vitamin A deficiency.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Moore S.W. Anorectal Malformations in Children. 2006th ed. Springer; Berlin/Heidelberg, Germany: 2006. Genetics, pathogenesis and epidemiology of anorectal malformations and caudal regression syndrome; pp. 31–48.
LinkOut - more resources
Full Text Sources

