CaFtsH06, A Novel Filamentous Thermosensitive Protease Gene, Is Involved in Heat, Salt, and Drought Stress Tolerance of Pepper (Capsicum annuum L.)
- PMID: 34203346
- PMCID: PMC8268771
- DOI: 10.3390/ijms22136953
CaFtsH06, A Novel Filamentous Thermosensitive Protease Gene, Is Involved in Heat, Salt, and Drought Stress Tolerance of Pepper (Capsicum annuum L.)
Abstract
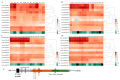
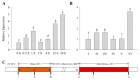
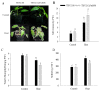
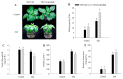
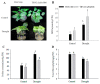
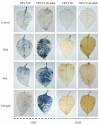
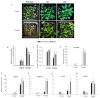
Harsh environmental factors have continuous negative effects on plant growth and development, leading to metabolic disruption and reduced plant productivity and quality. However, filamentation temperature-sensitive H protease (FtsH) plays a prominent role in helping plants to cope with these negative impacts. In the current study, we examined the transcriptional regulation of the CaFtsH06 gene in the R9 thermo-tolerant pepper (Capsicum annuum L.) line. The results of qRT-PCR revealed that CaFtsH06 expression was rapidly induced by abiotic stress treatments, including heat, salt, and drought. The CaFtsH06 protein was localized to the mitochondria and cell membrane. Additionally, silencing CaFtsH06 increased the accumulation of malonaldehyde content, conductivity, hydrogen peroxide (H2O2) content, and the activity levels of superoxide dismutase and superoxide (·O2-), while total chlorophyll content decreased under these abiotic stresses. Furthermore, CaFtsH06 ectopic expression enhanced tolerance to heat, salt, and drought stresses, thus decreasing malondialdehyde, proline, H2O2, and ·O2- contents while superoxide dismutase activity and total chlorophyll content were increased in transgenic Arabidopsis. Similarly, the expression levels of other defense-related genes were much higher in the transgenic ectopic expression lines than WT plants. These results suggest that CaFtsH06 confers abiotic stress tolerance in peppers by interfering with the physiological indices through reducing the accumulation of reactive oxygen species, inducing the activities of stress-related enzymes and regulating the transcription of defense-related genes, among other mechanisms. The results of this study suggest that CaFtsH06 plays a very crucial role in the defense mechanisms of pepper plants to unfavorable environmental conditions and its regulatory network with other CaFtsH genes should be examined across variable environments.
Keywords: CaFtsH06; Capsicum annuum L.; ROS-scavenging system; abiotic stress; transgenic Arabidopsis.
Conflict of interest statement
The authors declare there are no conflict of interest.
Figures






References
-
- Zhai Y., Wang H., Liang M., Lu M. Both silencing-and over-expression of pepper CaATG8c gene compromise plant tolerance to heat and salt stress. Environ. Exp. Bot. 2017;141:10–18. doi: 10.1016/j.envexpbot.2017.06.009. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

