Discovery and Heterologous Production of New Cyclic Depsibosamycins
- PMID: 34203385
- PMCID: PMC8303602
- DOI: 10.3390/microorganisms9071396
Discovery and Heterologous Production of New Cyclic Depsibosamycins
Abstract
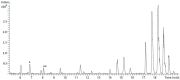
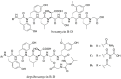
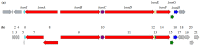
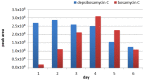
Streptomyces are producers of valuable secondary metabolites with unique scaffolds that perform a plethora of biological functions. Nonribosomal peptides are of special interest due to their variety and complexity. They are synthesized by nonribosomal peptide synthetases, large biosynthetic machineries that are encoded in the genome of many Streptomyces species. The identification of new peptides and the corresponding biosynthetic gene clusters is of major interest since knowledge can be used to facilitate combinatorial biosynthesis and chemical semisynthesis of natural products. The recently discovered bosamycins are linear octapeptides with an interesting 5-OMe tyrosine moiety and various modifications at the N-terminus. In this study, the new cyclic depsibosamycins B, C, and D from Streptomyces aurantiacus LU19075 were discovered. In comparison to the linear bosamycins B, C, and D, which were also produced by the strain, the cyclic depsibosamycins showed a side-chain-to-tail lactonization of serine and glycine, leading to a ring of four amino acids. In silico identification and heterologous expression of the depsibosamycin (dbm) gene cluster indicated that the cyclic peptides, rather than the linear derivatives, are the main products of the cluster.
Keywords: NRPS; Streptomyces; bosamycin; cyclic peptide; heterologous expression.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Bozhüyük K.A.J., Linck A., Tietze A., Kranz J., Wesche F., Nowak S., Fleischhacker F., Shi Y.-N., Grün P., Bode H.B. Modification and de novo design of non-ribosomal peptide synthetases using specific assembly points within condensation domains. Nat. Chem. 2019;11:653–661. doi: 10.1038/s41557-019-0276-z. - DOI - PubMed
LinkOut - more resources
Full Text Sources

