On the Coupling between Mechanical Properties and Electrostatics in Biological Membranes
- PMID: 34203412
- PMCID: PMC8306103
- DOI: 10.3390/membranes11070478
On the Coupling between Mechanical Properties and Electrostatics in Biological Membranes
Abstract
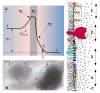
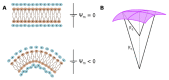
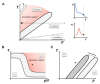
Cell membrane structure is proposed as a lipid matrix with embedded proteins, and thus, their emerging mechanical and electrostatic properties are commanded by lipid behavior and their interconnection with the included and absorbed proteins, cytoskeleton, extracellular matrix and ionic media. Structures formed by lipids are soft, dynamic and viscoelastic, and their properties depend on the lipid composition and on the general conditions, such as temperature, pH, ionic strength and electrostatic potentials. The dielectric constant of the apolar region of the lipid bilayer contrasts with that of the polar region, which also differs from the aqueous milieu, and these changes happen in the nanometer scale. Besides, an important percentage of the lipids are anionic, and the rest are dipoles or higher multipoles, and the polar regions are highly hydrated, with these water molecules forming an active part of the membrane. Therefore, electric fields (both, internal and external) affects membrane thickness, density, tension and curvature, and conversely, mechanical deformations modify membrane electrostatics. As a consequence, interfacial electrostatics appears as a highly important parameter, affecting the membrane properties in general and mechanical features in particular. In this review we focus on the electromechanical behavior of lipid and cell membranes, the physicochemical origin and the biological implications, with emphasis in signal propagation in nerve cells.
Keywords: electric field; electro-mechanical properties; electroporation; flexoelectricity; lipid ionization; nerve impulse.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Israelachvili J.N. Intermolecular and Surface Forces. Academic Press; Cambridge, MA, USA: 2011.
-
- Montich G.G., Bustos M.M., Maggio B., Cumar F.A. Micropolarity of interfaces containing anionic and neutral glycosphingolipids as sensed by Merocyanine 540. Chem. Phys. Lipids. 1985;38:319–326. doi: 10.1016/0009-3084(85)90026-X. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

