BPC 157 Therapy and the Permanent Occlusion of the Superior Sagittal Sinus in Rat: Vascular Recruitment
- PMID: 34203464
- PMCID: PMC8301421
- DOI: 10.3390/biomedicines9070744
BPC 157 Therapy and the Permanent Occlusion of the Superior Sagittal Sinus in Rat: Vascular Recruitment
Abstract
We show the complex syndrome of the occluded superior sagittal sinus, brain swelling and lesions and multiple peripheral organs lesions in rat. Recovery goes centrally and peripherally, with the stable gastric pentadecapeptide BPC 157, which alleviated peripheral vascular occlusion disturbances, rapidly activating alternative bypassing pathways. Assessments were gross recording, venography, ECG, pressure, microscopy, biochemistry. The increased pressure in the superior sagittal sinus, portal and caval hypertension, aortal hypotension, arterial and venous thrombosis, severe brain swelling and lesions (cortex (cerebral, cerebellar), hypothalamus/thalamus, hippocampus), particular veins (azygos, superior mesenteric, inferior caval) dysfunction, heart dysfunction, lung congestion as acute respiratory distress syndrome, kidney disturbances, liver failure, and hemorrhagic lesions in gastrointestinal tract were all assessed. Rats received BPC 157 medication (10 µg/kg, 10 ng/kg) intraperitoneally, intragastrically, or topically to the swollen brain at 1 min ligation-time, or at 15 min, 24 h and 48 h ligation-time. BPC 157 therapy rapidly attenuates the brain swelling, rapidly eliminates the increased pressure in the ligated superior sagittal sinus and the severe portal and caval hypertension and aortal hypotension, and rapidly recruits collateral vessels, centrally ((para)sagittal venous collateral circulation) and peripherally (left superior caval vein azygos vein-inferior caval vein). In conclusion, as shown by all assessments, BPC 157 acts against the permanent occlusion of the superior sagittal sinus and syndrome (i.e., brain, heart, lung, liver, kidney, gastrointestinal lesions, thrombosis), given at 1 min, 15 min, 24 h or 48 h ligation-time. BPC 157 therapy rapidly overwhelms the permanent occlusion of the superior sagittal sinus in rat.
Keywords: BPC 157; occlusion; rats; superior sagittal sinus; therapy; vascular recruitment.
Conflict of interest statement
The authors declare that there are no conflict of interest.
Figures


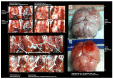

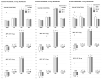
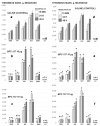



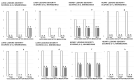





Similar articles
-
Occluded Superior Mesenteric Artery and Vein. Therapy with the Stable Gastric Pentadecapeptide BPC 157.Biomedicines. 2021 Jul 8;9(7):792. doi: 10.3390/biomedicines9070792. Biomedicines. 2021. PMID: 34356860 Free PMC article.
-
Occlusion of the Superior Mesenteric Artery in Rats Reversed by Collateral Pathways Activation: Gastric Pentadecapeptide BPC 157 Therapy Counteracts Multiple Organ Dysfunction Syndrome; Intracranial, Portal, and Caval Hypertension; and Aortal Hypotension.Biomedicines. 2021 May 26;9(6):609. doi: 10.3390/biomedicines9060609. Biomedicines. 2021. PMID: 34073625 Free PMC article.
-
Complex Syndrome of the Complete Occlusion of the End of the Superior Mesenteric Vein, Opposed with the Stable Gastric Pentadecapeptide BPC 157 in Rats.Biomedicines. 2021 Aug 17;9(8):1029. doi: 10.3390/biomedicines9081029. Biomedicines. 2021. PMID: 34440233 Free PMC article.
-
New studies with stable gastric pentadecapeptide protecting gastrointestinal tract. significance of counteraction of vascular and multiorgan failure of occlusion/occlusion-like syndrome in cytoprotection/organoprotection.Inflammopharmacology. 2024 Oct;32(5):3119-3161. doi: 10.1007/s10787-024-01499-8. Epub 2024 Jul 9. Inflammopharmacology. 2024. PMID: 38980576 Review.
-
Acute Compartment Syndrome and Intra-Abdominal Hypertension, Decompression, Current Pharmacotherapy, and Stable Gastric Pentadecapeptide BPC 157 Solution.Pharmaceuticals (Basel). 2025 Jun 10;18(6):866. doi: 10.3390/ph18060866. Pharmaceuticals (Basel). 2025. PMID: 40573261 Free PMC article. Review.
Cited by
-
Stable Gastric Pentadecapeptide BPC 157 and Striated, Smooth, and Heart Muscle.Biomedicines. 2022 Dec 12;10(12):3221. doi: 10.3390/biomedicines10123221. Biomedicines. 2022. PMID: 36551977 Free PMC article. Review.
-
Stable Gastric Pentadecapeptide BPC 157 Therapy: Effect on Reperfusion Following Maintained Intra-Abdominal Hypertension (Grade III and IV) in Rats.Pharmaceuticals (Basel). 2023 Nov 2;16(11):1554. doi: 10.3390/ph16111554. Pharmaceuticals (Basel). 2023. PMID: 38004420 Free PMC article.
-
Stable Gastric Pentadecapeptide BPC 157 May Counteract Myocardial Infarction Induced by Isoprenaline in Rats.Biomedicines. 2022 Jan 26;10(2):265. doi: 10.3390/biomedicines10020265. Biomedicines. 2022. PMID: 35203478 Free PMC article.
-
Novel Therapeutic Effects in Rat Spinal Cord Injuries: Recovery of the Definitive and Early Spinal Cord Injury by the Administration of Pentadecapeptide BPC 157 Therapy.Curr Issues Mol Biol. 2022 Apr 27;44(5):1901-1927. doi: 10.3390/cimb44050130. Curr Issues Mol Biol. 2022. PMID: 35678659 Free PMC article.
-
Therapy Effect of the Stable Gastric Pentadecapeptide BPC 157 on Acute Pancreatitis as Vascular Failure-Induced Severe Peripheral and Central Syndrome in Rats.Biomedicines. 2022 Jun 1;10(6):1299. doi: 10.3390/biomedicines10061299. Biomedicines. 2022. PMID: 35740321 Free PMC article.
References
-
- Sikiric P., Seiwerth S., Brcic L., Sever M., Klicek R., Radic B., Drmic D., Ilic S., Kolenc D. Revised Robert’s cytoprotection and adaptive cytoprotection and stable gastric pentadecapeptide BPC 157. Possible significance and implications for novel mediator. Curr. Pharm. Des. 2010;16:1224–1234. doi: 10.2174/138161210790945977. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

