Fracture Healing Research-Shift towards In Vitro Modeling?
- PMID: 34203470
- PMCID: PMC8301383
- DOI: 10.3390/biomedicines9070748
Fracture Healing Research-Shift towards In Vitro Modeling?
Abstract
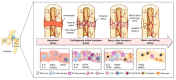
Fractures are one of the most frequently occurring traumatic events worldwide. Approximately 10% of fractures lead to bone healing disorders, resulting in strain for affected patients and enormous costs for society. In order to shed light into underlying mechanisms of bone regeneration (habitual or disturbed), and to develop new therapeutic strategies, various in vivo, ex vivo and in vitro models can be applied. Undeniably, in vivo models include the systemic and biological situation. However, transferability towards the human patient along with ethical concerns regarding in vivo models have to be considered. Fostered by enormous technical improvements, such as bioreactors, on-a-chip-technologies and bone tissue engineering, sophisticated in vitro models are of rising interest. These models offer the possibility to use human cells from individual donors, complex cell systems and 3D models, therefore bridging the transferability gap, providing a platform for the introduction of personalized precision medicine and finally sparing animals. Facing diverse processes during fracture healing and thus various scientific opportunities, the reliability of results oftentimes depends on the choice of an appropriate model. Hence, we here focus on categorizing available models with respect to the requirements of the scientific approach.
Keywords: bone tissue engineering; fracture healing; in vitro models; in vivo models.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the writing of the manuscript, or in the decision to publish the manuscript.
Figures
References
-
- Tzelepi V., Tsamandas A.C., Zolota V., Scopa C.D. Bone Anatomy, Physiology and Function. In: Kardamakis D., Vassiliou V., Chow E., editors. Bone Metastases: A Translational and Clinical Approach. Springer Netherlands; Dordrecht, The Netherlands: 2009. pp. 3–30.
-
- Cullinane D.M. The role of osteocytes in bone regulation: Mineral homeostasis versus mechanoreception. J. Musculoskelet. Neuronal Interact. 2002;2:242–244. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

