Cancer Cell Direct Bioprinting: A Focused Review
- PMID: 34203530
- PMCID: PMC8305105
- DOI: 10.3390/mi12070764
Cancer Cell Direct Bioprinting: A Focused Review
Abstract
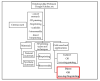
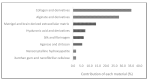
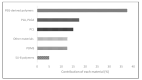
Three-dimensional printing technologies allow for the fabrication of complex parts with accurate geometry and less production time. When applied to biomedical applications, two different approaches, known as direct or indirect bioprinting, may be performed. The classical way is to print a support structure, the scaffold, and then culture the cells. Due to the low efficiency of this method, direct bioprinting has been proposed, with or without the use of scaffolds. Scaffolds are the most common technology to culture cells, but bioassembly of cells may be an interesting methodology to mimic the native microenvironment, the extracellular matrix, where the cells interact between themselves. The purpose of this review is to give an updated report about the materials, the bioprinting technologies, and the cells used in cancer research for breast, brain, lung, liver, reproductive, gastric, skin, and bladder associated cancers, to help the development of possible treatments to lower the mortality rates, increasing the effectiveness of guided therapies. This work introduces direct bioprinting to be considered as a key factor above the main tissue engineering technologies.
Keywords: 3D printing; bioprinting; cancer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Gabriel S., Hull C.W. Apparatus for Production of Three-Dimensional Objects by Stereolithography. U.S. Patent 4575330A. 1984 Mar 11;
-
- Hafezi F., Kucukgul C., Ozler S., Koc B. Additive Manufacturing. CRC Press; Boca Raton, FL, USA: 2015. Bioprinting: Application of Additive Manufacturing in Medicine; pp. 197–214.
-
- Mullen L., Stamp R.C., Brooks W.K., Jones E., Sutcliffe C.J. Selective laser melting: A regular unit cell approach for the manufacture of porous, titanium, bone in-growth constructs, suitable for orthopedic applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009;89:325–334. doi: 10.1002/jbm.b.31219. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

