Ca+ Ions Solvated in Helium Clusters
- PMID: 34203679
- PMCID: PMC8232145
- DOI: 10.3390/molecules26123642
Ca+ Ions Solvated in Helium Clusters
Abstract
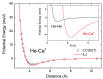
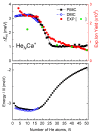
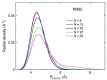
We present a combined experimental and theoretical investigation on Ca+ ions in helium droplets, HeNCa+. The clusters have been formed in the laboratory by means of electron-impact ionization of Ca-doped helium nanodroplets. Energies and structures of such complexes have been computed using various approaches such as path integral Monte Carlo, diffusion Monte Carlo and basin-hopping methods. The potential energy functions employed in these calculations consist of analytical expressions following an improved Lennard-Jones formula whose parameters are fine-tuned by exploiting ab initio estimations. Ion yields of HeNCa+ -obtained via high-resolution mass spectrometry- generally decrease with N with a more pronounced drop between N=17 and N=25, the computed quantum HeNCa+ evaporation energies resembling this behavior. The analysis of the energies and structures reveals that covering Ca+ with 17 He atoms leads to a cluster with one of the smallest energies per atom. As new atoms are added, they continue to fill the first shell at the expense of reducing its stability, until N=25, which corresponds to the maximum number of atoms in that shell. Behavior of the evaporation energies and radial densities suggests liquid-like cluster structures.
Keywords: classical/quantum monte carlo calculations; helium nanodroplets; helium-alkaline earth ion interactions; mass spectrometry; molecular clusters; solvation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Johnson W.W., Glaberson W.I. Positive impurity ions in He II. Phys. Rev. Lett. 1972;29:214–217. doi: 10.1103/PhysRevLett.29.214. - DOI
-
- Atkins K.R. Ions in Liquid Helium. Phys. Rev. 1959;116:1339–1343. doi: 10.1103/PhysRev.116.1339. - DOI
-
- Cole M.W., Bachman R.A. Structure of positive impurity ions in liquid helium. Phys. Rev. B. 1977;15:1388–1394. doi: 10.1103/PhysRevB.15.1388. - DOI
-
- Foerste M., Guenther H., Riediger O., Wiebe J., zu Putlitz G. Ions and atoms in superfluid helium (4He) Z. Phys. B Cond. Matt. 1997;104:317–322. doi: 10.1007/s002570050456. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

