Potential of Naturally Derived Compounds in Telomerase and Telomere Modulation in Skin Senescence and Aging
- PMID: 34203694
- PMCID: PMC8232155
- DOI: 10.3390/ijms22126381
Potential of Naturally Derived Compounds in Telomerase and Telomere Modulation in Skin Senescence and Aging
Abstract
Proper functioning of cells-their ability to divide, differentiate, and regenerate-is dictated by genomic stability. The main factors contributing to this stability are the telomeric ends that cap chromosomes. Telomere biology and telomerase activity have been of interest to scientists in various medical science fields for years, including the study of both cancer and of senescence and aging. All these processes are accompanied by telomere-length modulation. Maintaining the key levels of telomerase component (hTERT) expression and telomerase activity that provide optimal telomere length as well as some nontelomeric functions represents a promising step in advanced anti-aging strategies, especially in dermocosmetics. Some known naturally derived compounds contribute significantly to telomere and telomerase metabolism. However, before they can be safely used, it is necessary to assess their mechanisms of action and potential side effects. This paper focuses on the metabolic potential of natural compounds to modulate telomerase and telomere biology and thus prevent senescence and skin aging.
Keywords: antioxidants; natural compounds; senescence; skin aging; telomerase; telomeres.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
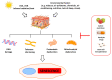

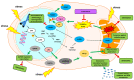
References
-
- Quan C., Cho M.K., Perry D., Quan T. Age-associated reduction of cell spreading induces mitochondrial DNA common deletion by oxidative stress in human skin dermal fibroblasts: Implication for human skin connective tissue aging. J. Biomed. Sci. 2015;22:62. doi: 10.1186/s12929-015-0167-6. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

