Pathophysiological Responses and Roles of Astrocytes in Traumatic Brain Injury
- PMID: 34203960
- PMCID: PMC8232783
- DOI: 10.3390/ijms22126418
Pathophysiological Responses and Roles of Astrocytes in Traumatic Brain Injury
Abstract
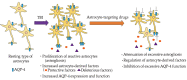
Traumatic brain injury (TBI) is immediate damage caused by a blow to the head resulting from traffic accidents, falls, and sporting activity, which causes death or serious disabilities in survivors. TBI induces multiple secondary injuries, including neuroinflammation, disruption of the blood-brain barrier (BBB), and brain edema. Despite these emergent conditions, current therapies for TBI are limited or insufficient in some cases. Although several candidate drugs exerted beneficial effects in TBI animal models, most of them failed to show significant effects in clinical trials. Multiple studies have suggested that astrocytes play a key role in the pathogenesis of TBI. Increased reactive astrocytes and astrocyte-derived factors are commonly observed in both TBI patients and experimental animal models. Astrocytes have beneficial and detrimental effects on TBI, including promotion and restriction of neurogenesis and synaptogenesis, acceleration and suppression of neuroinflammation, and disruption and repair of the BBB via multiple bioactive factors. Additionally, astrocytic aquaporin-4 is involved in the formation of cytotoxic edema. Thus, astrocytes are attractive targets for novel therapeutic drugs for TBI, although astrocyte-targeting drugs have not yet been developed. This article reviews recent observations of the roles of astrocytes and expected astrocyte-targeting drugs in TBI.
Keywords: astrogliosis; blood–brain barrier; cytotoxic edema; neuroinflammation; traumatic brain injury.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

