Enhanced Anticancer Activity of Nanoformulation of Dasatinib against Triple-Negative Breast Cancer
- PMID: 34204015
- PMCID: PMC8234460
- DOI: 10.3390/jpm11060559
Enhanced Anticancer Activity of Nanoformulation of Dasatinib against Triple-Negative Breast Cancer
Abstract
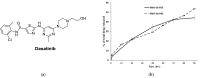
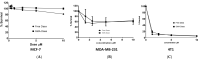
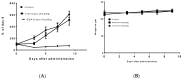
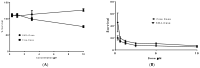
Triple negative breast cancer (TNBC) is the most aggressive breast cancer accounting for around 15% of identified breast cancer cases. TNBC lacks human epidermal growth factor receptor 2 (HER2) amplification, is hormone independent estrogen (ER) and progesterone receptors (PR) negative, and is not reactive to current targeted therapies. Existing treatment relies on chemotherapeutic treatment, but in spite of an initial response to chemotherapy, the inception of resistance and relapse is unfortunately common. Dasatinib is an approved second-generation inhibitor of multiple tyrosine kinases, and literature data strongly support its use in the management of TNBC. However, dasatinib binds to plasma proteins and undergoes extensive metabolism through oxidation and conjugation. To protect dasatinib from fast pharmacokinetic degradation and to prolong its activity, it was encapsulated on poly(styrene-co-maleic acid) (SMA) micelles. The obtained SMA-dasatinib nanoparticles (NPs) were evaluated for their physicochemical properties, in vitro antiproliferative activity in different TNBC cell lines, and in vivo anticancer activity in a syngeneic model of breast cancer. Obtained results showed that SMA-dasatinib is more potent against 4T1 TNBC tumor growth in vivo compared to free drug. This enhanced effect was ascribed to the encapsulation of the drug protecting it from a rapid metabolism. Our finding highlights the often-overlooked value of nanoformulations in protecting its cargo from degradation. Overall, results may provide an alternative therapeutic strategy for TNBC management.
Keywords: EPR; TNBC; dasatinib; metabolism; nanoformulation; nanomedicine; poly(styrene-co-maleic acid) micelles; targeted therapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

