Urine-Derived Epithelial Cells as Models for Genetic Kidney Diseases
- PMID: 34204173
- PMCID: PMC8230018
- DOI: 10.3390/cells10061413
Urine-Derived Epithelial Cells as Models for Genetic Kidney Diseases
Abstract
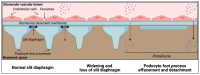
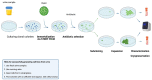
Epithelial cells exfoliated in human urine can include cells anywhere from the urinary tract and kidneys; however, podocytes and proximal tubular epithelial cells (PTECs) are by far the most relevant cell types for the study of genetic kidney diseases. When maintained in vitro, they have been proven extremely valuable for discovering disease mechanisms and for the development of new therapies. Furthermore, cultured patient cells can individually represent their human sources and their specific variants for personalized medicine studies, which are recently gaining much interest. In this review, we summarize the methodology for establishing human podocyte and PTEC cell lines from urine and highlight their importance as kidney disease cell models. We explore the well-established and recent techniques of cell isolation, quantification, immortalization and characterization, and we describe their current and future applications.
Keywords: Fanconi syndrome; PTECs; glomerular diseases; inherited renal disorders; kidney disease cellular models; podocytes; proximal tubular epithelial cells; renal tubular acidosis; urine-derived cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Elliott A.Y., Bronson D.L., Stein N., Fraley E.E. In Vitro Cultivation of Epithelial Cells Derived from Tumors of the Human Urinary Tract. Cancer Res. 1976;36:365–369. - PubMed
-
- Elliott A.Y., Bronson D.L., Cervenka J., Stein N., Fraley E.E. Properties of Cell Lines Established from Transitional Cell Cancers of the Human Urinary Tract. Cancer Res. 1977;37:1279–1289. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

