Effects of Orexin B on Swine Granulosa and Endothelial Cells
- PMID: 34204547
- PMCID: PMC8235033
- DOI: 10.3390/ani11061812
Effects of Orexin B on Swine Granulosa and Endothelial Cells
Abstract
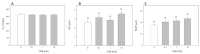
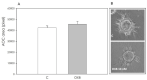
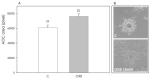
In addition to the well-known central modulatory role of orexins, we recently demonstrated a peripheral involvement in swine granulosa cells for orexin A and in adipose tissue for orexin B (OXB). The aim of present research was to verify immunolocalization of OXB and its potential role in modulating the main features of swine granulosa cells. In particular, we explored the effects on granulosa cell proliferation (through the incorporation of bromodeoxyuridine), cell metabolic activity (as indirect evaluation by the assessment of ATP), steroidogenic activity (by immunoenzymatic examination) and redox status (evaluating the production of superoxide anion by means of the WST test, production of nitric oxide through the use of the Griess test and the non-enzymatic reducing power by FRAP test). Our data point out that OXB does not modify granulosa cell growth, steroidogenesis and superoxide anion generation. On the contrary, the peptide stimulates (p < 0.05) nitric oxide output and non-enzymatic reducing power. Since new vessel growth is crucial for ovarian follicle development, a further aim of this study was to explore the expression of prepro-orexin and the effects of OXB on swine aortic endothelial cells. We found that the peptide is ineffective in modulating cell growth, while it inhibits redox status parameters. In addition, we demonstrated a stimulatory effect on angiogenesis evaluated in fibrin gel angiogenesis assay. Taken together, OXB appears to be potentially involved in the modulation of redox status in granulosa and endothelial cells and we could argue an involvement of the peptide in the follicular angiogenic events.
Keywords: angiogenesis; estradiol 17 β; ovarian follicle; oxidative stress; progesterone.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- de Lecea L., Kilduff T.S., Peyron C., Gao X., Foye P.E., Danielson P.E., Fukuhara C., Battenberg E.L., Gautvik V.T., Bartlett F.S., 2nd, et al. The hypocretins: Hypothalamus-specific peptides with neuroexcitatory activity. Proc. Natl. Acad. Sci. USA. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. - DOI - PMC - PubMed
-
- Sakurai T., Amemiya A., Ishii M., Matsuzaki I., Chemelli R.M., Tanaka H., Williams S.C., Richardson J.A., Kozlowski G.P., Wilson S., et al. Orexins and orexin receptors: A family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/S0092-8674(00)80949-6. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

