Hydrogen Sulfide Metabolism and Pulmonary Hypertension
- PMID: 34204699
- PMCID: PMC8231487
- DOI: 10.3390/cells10061477
Hydrogen Sulfide Metabolism and Pulmonary Hypertension
Abstract
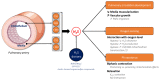
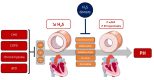
Pulmonary hypertension (PH) is a severe and multifactorial disease characterized by a progressive elevation of pulmonary arterial resistance and pressure due to remodeling, inflammation, oxidative stress, and vasoreactive alterations of pulmonary arteries (PAs). Currently, the etiology of these pathological features is not clearly understood and, therefore, no curative treatment is available. Since the 1990s, hydrogen sulfide (H2S) has been described as the third gasotransmitter with plethoric regulatory functions in cardiovascular tissues, especially in pulmonary circulation. Alteration in H2S biogenesis has been associated with the hallmarks of PH. H2S is also involved in pulmonary vascular cell homeostasis via the regulation of hypoxia response and mitochondrial bioenergetics, which are critical phenomena affected during the development of PH. In addition, H2S modulates ATP-sensitive K+ channel (KATP) activity, and is associated with PA relaxation. In vitro or in vivo H2S supplementation exerts antioxidative and anti-inflammatory properties, and reduces PA remodeling. Altogether, current findings suggest that H2S promotes protective effects against PH, and could be a relevant target for a new therapeutic strategy, using attractive H2S-releasing molecules. Thus, the present review discusses the involvement and dysregulation of H2S metabolism in pulmonary circulation pathophysiology.
Keywords: hydrogen sulfide; inflammation; oxidative stress; pulmonary hypertension; vascular reactivity; vascular remodeling.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Galiè N., Humbert M., Vachiéry J.-L., Gibbs S., Lang I.M., Kaminski K.A., Simonneau G., Peacock A., Noordegraaf A.V., Beghetti M., et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT) Eur. Heart J. 2015;37:67–119. doi: 10.1093/eurheartj/ehv317. - DOI - PubMed
-
- Noordegraaf A.V., Chin K.M., Haddad F., Hassoun P.M., Hemnes A.R., Hopkins S.R., Kawut S.M., Langleben D., Lumens J., Naeije R. Pathophysiology of the right ventricle and of the pulmonary circulation in pulmonary hypertension: An update. Eur. Respir. J. 2019;53:1801900. doi: 10.1183/13993003.01900-2018. - DOI - PMC - PubMed
-
- Humbert M., Guignabert C., Bonnet S., Dorfmüller P., Klinger J.R., Nicolls M.R., Olschewski A.J., Pullamsetti S.S., Schermuly R.T., Stenmark K.R., et al. Pathology and pathobiology of pulmonary hypertension: State of the art and research perspectives. Eur. Respir. J. 2019;53:1801887. doi: 10.1183/13993003.01887-2018. - DOI - PMC - PubMed
-
- De La Roque E.D., Smeralda G., Quignard J.-F., Freund-Michel V., Courtois A., Marthan R., Muller B., Guibert C., Dubois M. Altered vasoreactivity in neonatal rats with pulmonary hypertension associated with bronchopulmonary dysplasia: Implication of both eNOS phosphorylation and calcium signaling. PLoS ONE. 2017;12:e0173044. doi: 10.1371/journal.pone.0173044. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

