Impact of Hydrogen Peroxide on Protein Synthesis in Yeast
- PMID: 34204720
- PMCID: PMC8231629
- DOI: 10.3390/antiox10060952
Impact of Hydrogen Peroxide on Protein Synthesis in Yeast
Abstract
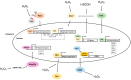
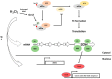
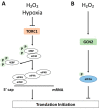
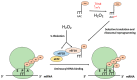
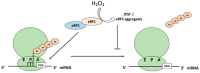
Cells must be able to respond and adapt to different stress conditions to maintain normal function. A common response to stress is the global inhibition of protein synthesis. Protein synthesis is an expensive process consuming much of the cell's energy. Consequently, it must be tightly regulated to conserve resources. One of these stress conditions is oxidative stress, resulting from the accumulation of reactive oxygen species (ROS) mainly produced by the mitochondria but also by other intracellular sources. Cells utilize a variety of antioxidant systems to protect against ROS, directing signaling and adaptation responses at lower levels and/or detoxification as levels increase to preclude the accumulation of damage. In this review, we focus on the role of hydrogen peroxide, H2O2, as a signaling molecule regulating protein synthesis at different levels, including transcription and various parts of the translation process, e.g., initiation, elongation, termination and ribosome recycling.
Keywords: cysteine thiols; hydrogen peroxide; protein synthesis; signaling.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Davies K.J. Oxidative stress: The paradox of aerobic life. Biochem. Soc. Symp. 1995;61:1–31. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

