Heat-Labile Toxin from Enterotoxigenic Escherichia coli Causes Systemic Impairment in Zebrafish Model
- PMID: 34204819
- PMCID: PMC8231604
- DOI: 10.3390/toxins13060419
Heat-Labile Toxin from Enterotoxigenic Escherichia coli Causes Systemic Impairment in Zebrafish Model
Abstract
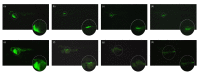
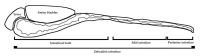
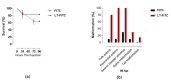
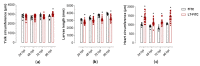
Heat-labile toxin I (LT-I), produced by strains of enterotoxigenic Escherichia coli (ETEC), causes profuse watery diarrhea in humans. Different in vitro and in vivo models have already elucidated the mechanism of action of this toxin; however, their use does not always allow for more specific studies on how the LT-I toxin acts in systemic tracts and intestinal cell lines. In the present work, zebrafish (Danio rerio) and human intestinal cells (Caco-2) were used as models to study the toxin LT-I. Caco-2 cells were used, in the 62nd passage, at different cell concentrations. LT-I was conjugated to FITC to visualize its transport in cells, as well as microinjected into the caudal vein of zebrafish larvae, in order to investigate its effects on survival, systemic traffic, and morphological formation. The internalization of LT-I was visualized in 3 × 104 Caco-2 cells, being associated with the cell membrane and nucleus. The systemic traffic of LT-I in zebrafish larvae showed its presence in the cardiac cavity, yolk, and regions of the intestine, as demonstrated by cardiac edema (100%), the absence of a swimming bladder (100%), and yolk edema (80%), in addition to growth limitation in the larvae, compared to the control group. There was a reduction in heart rate during the assessment of larval survival kinetics, demonstrating the cardiotoxic effect of LT-I. Thus, in this study, we provide essential new depictions of the features of LT-I.
Keywords: Caco-2 cells; cardiotoxic effect; heat-labile toxin; systemic effects; zebrafish.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Schaechter M., Medoff G., Eisenstein B.I. Mechanisms of Microbial Disease. 2nd ed. Williams and Wilkins; Baltimore, MD, USA: 1993. pp. 162–175.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

