Diversity in Chemical Structures and Biological Properties of Plant Alkaloids
- PMID: 34204857
- PMCID: PMC8199754
- DOI: 10.3390/molecules26113374
Diversity in Chemical Structures and Biological Properties of Plant Alkaloids
Abstract
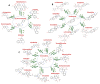
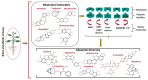
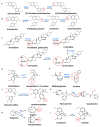
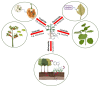
Phytochemicals belonging to the group of alkaloids are signature specialized metabolites endowed with countless biological activities. Plants are armored with these naturally produced nitrogenous compounds to combat numerous challenging environmental stress conditions. Traditional and modern healthcare systems have harnessed the potential of these organic compounds for the treatment of many ailments. Various chemical entities (functional groups) attached to the central moiety are responsible for their diverse range of biological properties. The development of the characterization of these plant metabolites and the enzymes involved in their biosynthesis is of an utmost priority to deliver enhanced advantages in terms of biological properties and productivity. Further, the incorporation of whole/partial metabolic pathways in the heterologous system and/or the overexpression of biosynthetic steps in homologous systems have both become alternative and lucrative methods over chemical synthesis in recent times. Moreover, in-depth research on alkaloid biosynthetic pathways has revealed numerous chemical modifications that occur during alkaloidal conversions. These chemical reactions involve glycosylation, acylation, reduction, oxidation, and methylation steps, and they are usually responsible for conferring the biological activities possessed by alkaloids. In this review, we aim to discuss the alkaloidal group of plant specialized metabolites and their brief classification covering major categories. We also emphasize the diversity in the basic structures of plant alkaloids arising through enzymatically catalyzed structural modifications in certain plant species, as well as their emerging diverse biological activities. The role of alkaloids in plant defense and their mechanisms of action are also briefly discussed. Moreover, the commercial utilization of plant alkaloids in the marketplace displaying various applications has been enumerated.
Keywords: alkaloid; biological activity; classification; defense; enzyme; modification.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Pott D.M., Osorio S., Vallarino J.G. From central to specialized metabolism: An overview of some secondary compounds derived from the primary metabolism for their role in conferring nutritional and organoleptic characteristics to fruit. Front. Plant Sci. 2019;10:835. doi: 10.3389/fpls.2019.00835. - DOI - PMC - PubMed
-
- Kuramoto M., Arimoto H., Uemura D. Bioactive alkaloids from the sea: A review. Mar. Drugs. 2004;2:39–54. doi: 10.3390/md201039. - DOI
-
- Braekman J.C., Daloze D., Pasteels J.M. Alkaloids in animals. In: Roberts M.F., Wink M., editors. Alkaloids: Biochemistry, ecology, and medicinal applications. Plenum Press; New York, NY, USA: 1998. pp. 349–378.
-
- Cárdenas P.D., Sonawane P.D., Heinig U., Heinig U., Jozwiak A., Panda S., Abebie B., Kazachkova Y., Pliner M., Unger T., et al. Pathways to defense metabolites and evading fruit bitterness in genus Solanum evolved through 2-oxoglutarate-dependent dioxygenases. Nat. Commun. 2019;10:5169. doi: 10.1038/s41467-019-13211-4. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- Focused Basic Research (FBR) project (MLP0007) - Genome-editing for Crop Improvement (GE-Crop)/Council of Scientific and Industrial Research, New Delhi, Government of India
- 31/11(1083)/2019-EMR-I/Council of Scientific and Industrial Research (CSIR), India
- IFA18-LSPA 123/Department of Science and Technology (DST) - Inspire Faculty program (India)
LinkOut - more resources
Full Text Sources

