Improving Phage-Biofilm In Vitro Experimentation
- PMID: 34205417
- PMCID: PMC8234374
- DOI: 10.3390/v13061175
Improving Phage-Biofilm In Vitro Experimentation
Abstract
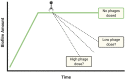
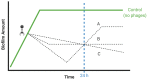
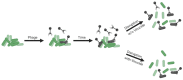
Bacteriophages or phages, the viruses of bacteria, are abundant components of most ecosystems, including those where bacteria predominantly occupy biofilm niches. Understanding the phage impact on bacterial biofilms therefore can be crucial toward understanding both phage and bacterial ecology. Here, we take a critical look at the study of bacteriophage interactions with bacterial biofilms as carried out in vitro, since these studies serve as bases of our ecological and therapeutic understanding of phage impacts on biofilms. We suggest that phage-biofilm in vitro experiments often may be improved in terms of both design and interpretation. Specific issues discussed include (a) not distinguishing control of new biofilm growth from removal of existing biofilm, (b) inadequate descriptions of phage titers, (c) artificially small overlying fluid volumes, (d) limited explorations of treatment dosing and duration, (e) only end-point rather than kinetic analyses, (f) importance of distinguishing phage enzymatic from phage bacteriolytic anti-biofilm activities, (g) limitations of biofilm biomass determinations, (h) free-phage interference with viable-count determinations, and (i) importance of experimental conditions. Toward bettering understanding of the ecology of bacteriophage-biofilm interactions, and of phage-mediated biofilm disruption, we discuss here these various issues as well as provide tips toward improving experiments and their reporting.
Keywords: bacteriophage therapy; biocontrol; biofilm; biofouling; biological control; chronic infection; microcolony; phage ecology; phage therapy.
Conflict of interest statement
S.T.A. has consulted for and served on advisory boards for companies with phage therapy interests, holds an equity stake in a number of these companies, and maintains the websites
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

