Evaluation of SARS-CoV-2 Spike Protein Antibody Titers in Cord Blood after COVID-19 Vaccination during Pregnancy in Polish Healthcare Workers: Preliminary Results
- PMID: 34205434
- PMCID: PMC8234119
- DOI: 10.3390/vaccines9060675
Evaluation of SARS-CoV-2 Spike Protein Antibody Titers in Cord Blood after COVID-19 Vaccination during Pregnancy in Polish Healthcare Workers: Preliminary Results
Abstract
Background: The coronavirus disease 2019 (COVID-19) pandemic has given rise to the need to develop a vaccine as quickly as possible. As pregnant women are at increased risk of contracting severe COVID-19, with higher mortality, it is essential to assess the safety of the vaccines administered during pregnancy.
Methods: The aim of this study was to determine the titer of specific maternal and cord antibodies against severe acute respiratory syndrome coronavirus 2 S protein after antenatal vaccination. The secondary objective was to evaluate the ratio of the umbilical cord to the maternal antibody titers. Patients included in the study were enrolled after undergoing voluntary vaccination against COVID-19 during pregnancy at different weeks of gestation. All patients analyzed in our initial study were vaccinated with the BNT162b2 mRNA COVID-19 vaccine.
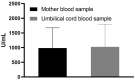
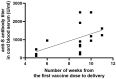
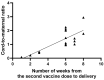
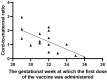
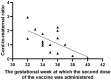
Results: The results of the current study document high anti-S total IgG antibody titers in cord serum at birth in all mother-infant pairs analyzed. The mean umbilical cord blood sample IgG antibody titer anti-S protein was 1026.51 U/mL (±SD 769.25). The mean cord-to-maternal anti-S IgG antibody ratio was 1.28 (±SD 0.798). A significant positive correlation was observed between the week of gestation at which the first dose was administered and the week of gestation at which the second dose was administered, and the respective cord-to-maternal ratio (r = 0.48; p = 0.0029) for the first dose and (r = 0.39; p = 0.0102) for the second dose.
Conclusions: To date, despite the prevalence of COVID-19 vaccination, there is a lack of conclusive evidence supporting the safety and efficacy of vaccination of pregnant women. Therefore, the results we present are complementary. Our study suggests that maternal immunization may provide neonatal protection through the transplacental transfer of antibodies. Of particular importance is the demonstration that antibody transfer is correlated with the time from vaccination to delivery, which may allow future determination of the optimal timing of COVID-19 vaccination in pregnant women.
Keywords: COVID-19; pregnancy; vaccine.
Conflict of interest statement
The authors declare no potential conflict of interest.
Figures


References
-
- Masmejan S., Pomar L., Lepigeon K., Favre G., Baud D., Rieder W. COVID-19 et grossesse [COVID-19 and pregnancy] Rev. Med. Suisse. 2020;16:944–946. - PubMed
-
- European Medicines Agency Procedure No. EMEA/H/C/005735/0000. Assessment Report: COVID-19 mRNA Vaccine (Nucleoside-Modified) [(accessed on 6 April 2021)]; Available online: https://www.ema.europa.eu/en/documents/assessment-report/comirnaty-epar-....
-
- COVID-19 (Coronavirus Disease): People with Certain Medical Conditions. [(accessed on 6 April 2021)]; Available online: https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-....
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

