Antimicrobial Use in COVID-19 Patients in the First Phase of the SARS-CoV-2 Pandemic: A Scoping Review
- PMID: 34205440
- PMCID: PMC8235357
- DOI: 10.3390/antibiotics10060745
Antimicrobial Use in COVID-19 Patients in the First Phase of the SARS-CoV-2 Pandemic: A Scoping Review
Abstract
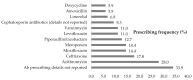
This scoping review provides new evidence on the prevalence and patterns of global antimicrobial use in the treatment of COVID-19 patients; identifies the most commonly used antibiotics and clinical scenarios associated with antibiotic prescribing in the first phase of the pandemic; and explores the impact of documented antibiotic prescribing on treatment outcomes in COVID-19 patients. The review complies with PRISMA guidelines for Scoping Reviews and the protocol is registered with the Open Science Framework. In the first six months of the pandemic, there was a similar mean antibiotic prescribing rate between patients with severe or critical illness (75.4%) and patients with mild or moderate illness (75.1%). The proportion of patients prescribed antibiotics without clinical justification was 51.5% vs. 41.9% for patients with mild or moderate illness and those with severe or critical illness. Comparison of patients who were provided antibiotics with a clinical justification with those who were given antibiotics without clinical justification showed lower mortality rates (9.5% vs. 13.1%), higher discharge rates (80.9% vs. 69.3%), and shorter length of hospital stay (9.3 days vs. 12.2 days). In the first 6 months of the pandemic, antibiotics were prescribed for COVID-19 patients regardless of severity of illness. A large proportion of antibiotic prescribing for mild and moderate COVID-19 patients did not have clinical evidence of a bacterial co-infection. Antibiotics may not be beneficial to COVID-19 patients without clinical evidence of a bacterial co-infection.
Keywords: COVID-19 patients; antibiotic use; clinical justification; disease severity; secondary infections.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- WHO No Time to Wait: Securing the Future from Drug-Resistant Infections. [(accessed on 4 December 2020)];Report to the Secretary-General of the United Nations (IACG). Available online: https://www.who.int/publications/i/item/no-time-to-wait-securing-the-fut....
-
- O’Neill J., Bretagne B. Antimicrobial Resistance: Tackling a Crisis for the Health and Wealth of Nations. Wellcome Trust; London, UK: 2014.
-
- Vaughn V.M., Gandhi T., Petty L.A., Patel P.K., Prescott H.C., Malani A.N., Ratz D., McLaughlin E., Chopra V., Flanders S.A. Empiric Antibacterial Therapy and Community-onset Bacterial Coinfection in Patients Hospitalized with COVID-19: A Multi-Hospital Cohort Study. Clin. Infect. Dis. 2021;72:e533–e541. doi: 10.1093/cid/ciaa1239. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous