NGS-Based Analysis of Atypical Deep Penetrating Nevi
- PMID: 34205480
- PMCID: PMC8234376
- DOI: 10.3390/cancers13123066
NGS-Based Analysis of Atypical Deep Penetrating Nevi
Abstract
Deep penetrating nevi (DPNs) are rare melanocytic neoplasms consisting of pigmented spindled or epithelioid melanocytes with a distinctive wedge-shaped configuration showing activation of the WNT pathway, with unusual cyto-architectural features. It is unclear whether they show a distinct genomic profile associated with a diverse metastatic potential. We describe herein a cohort of 21 atypical DPNs analyzed by next-generation sequencing using the Ion AmpliSeq™ Comprehensive Cancer Panel. We found that β-catenin exon 3 was mutated in 95% and MAP kinase pathway genes in 71% of the cases. Less frequent mutations were observed in HRAS (19%) and MAP2K1 (24%). Isocitrate dehydrogenases 1 (IDH1) mutations, including R132C, V178I, and S278L, were identified in 38% of cases and co-existed with BRAF/HRAS mutations. The only case with progressive nodal disease carried alterations in the β-catenin pathway and mutations in IDH1 and NRAS (codon 61). By a comprehensive mutation analysis, we found low genetic heterogeneity and a lack of significant associations between specific gene mutations and histopathological features, despite atypical features. Whether the acquisition of an NRAS or IDH1 mutation in an atypical DPN may represent a molecular evolution implying a pathway to melanoma progression should be confirmed in a larger series.
Keywords: atypical deep penetrating nevus; borderline/atypical deep penetrating nevus; deep penetrating nevus (DPN); next-generation sequencing (NGS); β-catenin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

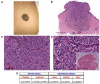
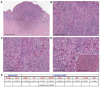
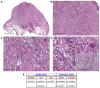

References
-
- Robson A., Morley-Quante M., Hempel H., McKee P.H., Calonje E. Deep penetrating naevus: Clinicopathological study of 31 cases with further delineation of histological features allowing distinction from other pigmented benign melanocytic lesions and melanoma. Histopathology. 2003;43:529–537. doi: 10.1111/j.1365-2559.2003.01730.x. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

