Microbial Oligosaccharides with Biomedical Applications
- PMID: 34205503
- PMCID: PMC8234114
- DOI: 10.3390/md19060350
Microbial Oligosaccharides with Biomedical Applications
Abstract
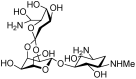
Microbial oligosaccharides have been regarded as one of the most appealing natural products attributable to their potent and selective bioactivities, such as antimicrobial activity, inhibition of α-glucosidases and lipase, interference of cellular recognition and signal transduction, and disruption of cell wall biosynthesis. Accordingly, a handful of bioactive oligosaccharides have been developed for the treatment of bacterial infections and type II diabetes mellitus. Given that naturally occurring oligosaccharides have increasingly gained recognition in recent years, a comprehensive review is needed. The current review highlights the chemical structures, biological activities and divergent biosynthetic origins of three subgroups of oligomers including the acarviosine-containing oligosaccharides, saccharomicins, and orthosomycins.
Keywords: aminooligosaccharide; biomedical applications; biosynthesis; chemical structure; microbial oligosaccharide; orthosomycin; saccharomicin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures










References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

