Electrophysiology of hiPSC-Cardiomyocytes Co-Cultured with HEK Cells Expressing the Inward Rectifier Channel
- PMID: 34205607
- PMCID: PMC8235371
- DOI: 10.3390/ijms22126621
Electrophysiology of hiPSC-Cardiomyocytes Co-Cultured with HEK Cells Expressing the Inward Rectifier Channel
Abstract
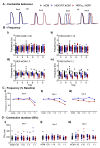
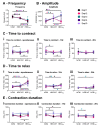
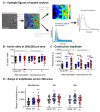
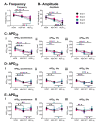
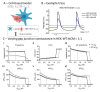
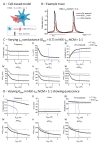
The immature electrophysiology of human-induced pluripotent stem cell-derived cardiomyocytes (hiCMs) complicates their use for therapeutic and pharmacological purposes. An insufficient inward rectifying current (IK1) and the presence of a funny current (if) cause spontaneous electrical activity. This study tests the hypothesis that the co-culturing of hiCMs with a human embryonic kidney (HEK) cell-line expressing the Kir2.1 channel (HEK-IK1) can generate an electrical syncytium with an adult-like cardiac electrophysiology. The mechanical activity of co-cultures using different HEK-IK1:hiCM ratios was compared with co-cultures using wildtype (HEK-WT:hiCM) or hiCM alone on days 3-8 after plating. Only ratios of 1:3 and 1:1 showed a significant reduction in spontaneous rate at days 4 and 6, suggesting that IK1 was influencing the electrophysiology. Detailed analysis at day 4 revealed an increased incidence of quiescent wells or sub-areas. Electrical activity showed a decreased action potential duration (APD) at 20% and 50%, but not at 90%, alongside a reduced amplitude of the aggregate AP signal. A computational model of the 1:1 co-culture replicates the electrophysiological effects of HEK-WT. The addition of the IK1 conductance reduced the spontaneous rate and APD20, 50 and 90, and minor variation in the intercellular conductance caused quiescence. In conclusion, a 1:1 co-culture HEK-IK1:hiCM caused changes in electrophysiology and spontaneous activity consistent with the integration of IK1 into the electrical syncytium. However, the additional electrical effects of the HEK cell at 1:1 increased the possibility of electrical quiescence before sufficient IK1 was integrated into the syncytium.
Keywords: HEK; IK1; co-culture; electrophysiology; hiCMs; maturation.
Conflict of interest statement
The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
References
-
- Verkerk A.O., Veerman C.C., Zegers J.G., Mengarelli I., Bezzina C.R., Wilders R. Patch-clamp recording from human induced pluripotent stem cell-derived cardiomyocytes: Improving action potential characteristics through dynamic clamp. Int. J. Mol. Sci. 2017;18:1873. doi: 10.3390/ijms18091873. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

