Functionalized Particles Designed for Targeted Delivery
- PMID: 34205672
- PMCID: PMC8234925
- DOI: 10.3390/polym13122022
Functionalized Particles Designed for Targeted Delivery
Abstract
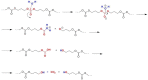
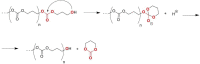
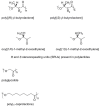
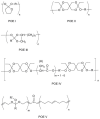
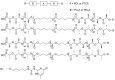
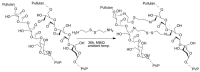
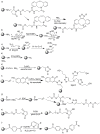
Pure bioactive compounds alone can only be exceptionally administered in medical treatment. Usually, drugs are produced as various forms of active compounds and auxiliary substances, combinations assuring the desired healing functions. One of the important drug forms is represented by a combination of active substances and particle-shaped polymer in the nano- or micrometer size range. The review describes recent progress in this field balanced with basic information. After a brief introduction, the paper presents a concise overview of polymers used as components of nano- and microparticle drug carriers. Thereafter, progress in direct synthesis of polymer particles with functional groups is discussed. A section is devoted to formation of particles by self-assembly of homo- and copolymer-bearing functional groups. Special attention is focused on modification of the primary functional groups introduced during particle preparation, including introduction of ligands promoting anchorage of particles onto the chosen living cell types by interactions with specific receptors present in cell membranes. Particular attention is focused on progress in methods suitable for preparation of particles loaded with bioactive substances. The review ends with a brief discussion of the still not answered questions and unsolved problems.
Keywords: functional polymer; microparticle; nanoparticle; nucleic acid; polymerosome; protein; targeted drug delivery.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
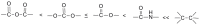
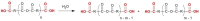

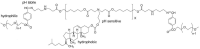

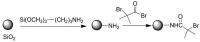
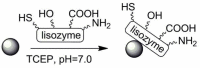
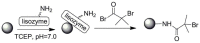

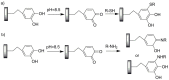
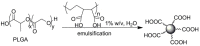
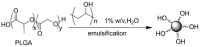
Similar articles
-
Progress in nanoparticulate systems for peptide, proteins and nucleic acid drug delivery.Curr Pharm Biotechnol. 2011 Nov;12(11):1823-39. doi: 10.2174/138920111798377003. Curr Pharm Biotechnol. 2011. PMID: 21902630 Review.
-
Chemical Approaches for the Preparation of Bacteria - Nano/Microparticle Hybrid Systems.Macromol Biosci. 2023 Aug;23(8):e2200440. doi: 10.1002/mabi.202200440. Epub 2022 Dec 15. Macromol Biosci. 2023. PMID: 36454518 Review.
-
Erratum: Preparation of Poly(pentafluorophenyl acrylate) Functionalized SiO2 Beads for Protein Purification.J Vis Exp. 2019 Apr 30;(146). doi: 10.3791/6328. J Vis Exp. 2019. PMID: 31038480
-
Functional Ligand-Enabled Particle Assembly for Bio-Nano Interactions.Acc Chem Res. 2023 Jul 4;56(13):1826-1837. doi: 10.1021/acs.accounts.3c00172. Epub 2023 May 24. Acc Chem Res. 2023. PMID: 37225704
-
Colloidal capsules: nano- and microcapsules with colloidal particle shells.Chem Soc Rev. 2017 Apr 18;46(8):2091-2126. doi: 10.1039/c6cs00632a. Chem Soc Rev. 2017. PMID: 28230870 Review.
Cited by
-
Synthesis, Characterization and In Vitro Evaluation of Chitosan Nanoparticles Physically Admixed with Lactose Microspheres for Pulmonary Delivery of Montelukast.Polymers (Basel). 2022 Aug 29;14(17):3564. doi: 10.3390/polym14173564. Polymers (Basel). 2022. PMID: 36080637 Free PMC article.
-
State-of-the-Art Advances and Current Applications of Gel-Based Membranes.Gels. 2024 Jan 1;10(1):39. doi: 10.3390/gels10010039. Gels. 2024. PMID: 38247761 Free PMC article. Review.
-
Biomineralization of Human Genomic DNA into ZIF-8, a Zeolite-Like Metal-Organic Framework.Sovrem Tekhnologii Med. 2024;16(1):5-13. doi: 10.17691/stm2024.16.1.01. Epub 2024 Feb 28. Sovrem Tekhnologii Med. 2024. PMID: 39421628 Free PMC article.
-
Application of Starch, Cellulose, and Their Derivatives in the Development of Microparticle Drug-Delivery Systems.Polymers (Basel). 2023 Aug 31;15(17):3615. doi: 10.3390/polym15173615. Polymers (Basel). 2023. PMID: 37688241 Free PMC article. Review.
-
Double Peptide-Functionalized Carboxymethyl Chitosan-Coated Liposomes Loaded with Dexamethasone as a Potential Strategy for Active Targeting Drug Delivery.Int J Mol Sci. 2025 Jan 22;26(3):922. doi: 10.3390/ijms26030922. Int J Mol Sci. 2025. PMID: 39940692 Free PMC article.
References
-
- Slomkowski S., Alemán J.V., Gilbert R.G., Hess M., Horie K., Jones R.G., Kubisa P., Meisel I., Mormann W., Penczek S., et al. Terminology of Polymers and Polymerization Processes in Dispersed Systems (IUPAC Recommendations 2011) Pure Appl. Chem. 2011;83:2229–2259. doi: 10.1351/PAC-REC-10-06-03. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

