Age and Infectious Dose Significantly Affect Disease Progression after RHDV2 Infection in Naïve Domestic Rabbits
- PMID: 34205750
- PMCID: PMC8234499
- DOI: 10.3390/v13061184
Age and Infectious Dose Significantly Affect Disease Progression after RHDV2 Infection in Naïve Domestic Rabbits
Abstract
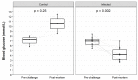
Rabbit haemorrhagic disease virus 2 (RHDV2 or GI.2, referring to any virus with lagovirus GI.2 structural genes) is a recently emerged calicivirus that causes generalised hepatic necrosis and disseminated intravascular coagulation leading to death in susceptible lagomorphs (rabbits and hares). Previous studies investigating the virulence of RHDV2 have reported conflicting results, with case fatality rates ranging from 0% to 100% even within a single study. Lagoviruses are of particular importance in Australia and New Zealand where they are used as biocontrol agents to manage wild rabbit populations, which threaten over 300 native species and result in economic impacts in excess of $200 million AUD annually to Australian agricultural industries. It is critically important that any pest control method is both highly effective (i.e., virulent, in the context of viral biocontrols) and has minimal animal welfare impacts. To determine whether RHDV2 might be a suitable candidate biocontrol agent, we investigated the virulence and disease progression of a naturally occurring Australian recombinant RHDV2 in both 5-week-old and 11-week-old New Zealand White laboratory rabbits after either high or low dose oral infection. Objective measures of disease progression were recorded through continuous body temperature monitoring collars, continuous activity monitors, and twice daily observations. We observed a 100% case fatality rate in both infected kittens and adult rabbits after either high dose or low dose infection. Clinical signs of disease, such as pyrexia, weight loss, and reduced activity, were evident in the late stages of infection. Clinical disease, i.e., welfare impacts, were limited to the period after the onset of pyrexia, lasting on average 12 h and increasing in severity as disease progressed. These findings confirm the high virulence of this RHDV2 variant in naïve rabbits. While age and infectious dose significantly affected disease progression, the case fatality rate was consistently 100% under all conditions tested.
Keywords: RHDV2; animal welfare; calicivirus; lagovirus; rabbit; rabbit haemorrhagic disease virus; virulence.
Conflict of interest statement
Funding for this work was provided through the Centre for Invasive Species Solutions to investigate RHDV2 as a potential additional biocontrol agent to manage invasive wild rabbits in Australia. The funding body and project lead did not have input into the experimental design, data analysis, or preparation of this manuscript.
Figures








References
-
- Liu S.J., Xue H.P., Pu B.Q., Qian N.H. A new viral disease in rabbits. J. Vet. Diagn. Invest. 1984;16:253–255.
-
- Spickler A. Rabbit Hemorrhagic Disease. [(accessed on 5 March 2021)];2016 Available online: https://www.cfsph.iastate.edu/Factsheets/pdfs/rabbit_hemorrhagic_disease....
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

