Advanced Maternal Age Deteriorates the Developmental Competence of Vitrified Oocytes in Mice
- PMID: 34205802
- PMCID: PMC8234289
- DOI: 10.3390/cells10061563
Advanced Maternal Age Deteriorates the Developmental Competence of Vitrified Oocytes in Mice
Abstract
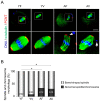
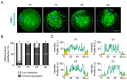
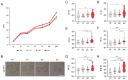
Advanced maternal age (AMA) is known to be related to the decrease in the quality and quantity of oocytes. Oocyte vitrification is now considered an established assisted reproductive technology for fertility preservation. However, it remains unclear whether the oocytes in older women are more sensitive to various insults during vitrification. Thus, we evaluated whether AMA affects cellular and molecular features and developmental outcomes of oocytes after vitrification in mice. The oocytes were grouped as young fresh (YF), young vitrified/warmed (YV), aged fresh (AF), and aged vitrified/warmed (AV). The survival rate of AV oocytes was significantly lower than that of YV oocytes. The rates of fertilization, cleavage, and blastocyst formation of AV oocytes were significantly lower than those of other groups. AV oocytes were represented as aberrations in mitochondria distribution, microvacuole size, and autophagosome formation, leading to delayed embryo development in mice. This delay was associated with a reduced number of total cells and trophectoderm in the blastocyst developed from AV oocytes. Collectively, AMA exaggerates the vulnerability of oocytes to cryo-damage that occurs during vitrification in mice, suggesting that the current vitrification protocols optimized for oocytes from young females should be modified for oocytes from aged women.
Keywords: advanced maternal age (AMA); developmental competence; fertility preservation; oocyte quality; oocyte vitrification; time-lapse monitoring.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Ubaldi F.M., Cimadomo D., Vaiarelli A., Fabozzi G., Venturella R., Maggiulli R., Mazzilli R., Ferrero S., Palagiano A., Rienzi L. Advanced maternal age in IVF: Still a challenge? The present and the future of its treatment. Front. Endocrinol. 2019;10:94. doi: 10.3389/fendo.2019.00094. - DOI - PMC - PubMed
-
- Johnston M., Richings N.M., Leung A., Sakkas D., Catt S. A major increase in oocyte cryopreservation cycles in the USA, Australia and New Zealand since 2010 is highlighted by younger women but a need for standardized data collection. Hum. Reprod. 2020;36:624–635. doi: 10.1093/humrep/deaa320. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

