T-Cell Lymphoblastic Lymphoma Arising in the Setting of Myeloid/Lymphoid Neoplasms with Eosinophilia: LMO2 Immunohistochemistry as a Potentially Useful Diagnostic Marker
- PMID: 34205834
- PMCID: PMC8234657
- DOI: 10.3390/cancers13123102
T-Cell Lymphoblastic Lymphoma Arising in the Setting of Myeloid/Lymphoid Neoplasms with Eosinophilia: LMO2 Immunohistochemistry as a Potentially Useful Diagnostic Marker
Abstract
Background: Rarely, T-lymphoblastic lymphoma (T-LBL) may develop in the setting of myeloid/lymphoid neoplasms with eosinophilia (M/LNs-Eo), a group of diseases with gene fusion resulting in overexpression of an aberrant tyrosine kinase or cytokine receptor. The correct identification of this category has relevant therapeutic implications. LIM domain only 2 (LMO2) is overexpressed in most T-LBL, but not in immature TdT-positive T-cells in the thymus and in indolent T-lymphoblastic proliferations (iT-LBP).
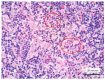
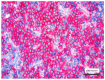
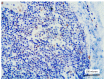
Methods and results: We retrospectively evaluated 11 cases of T-LBL occurring in the context of M/LNs-Eo. Clinical, histological, immunohistochemical and molecular features were collected and LMO2 immunohistochemical staining was performed. The critical re-evaluation of these cases confirmed the diagnosis of T-LBL with morphological, immunohistochemical and molecular features consistent with T-LBL occurring in M/LNs-Eo. Interestingly, LMO2 immunohistochemical analysis was negative in 9/11 cases, whereas only 2 cases revealed a partial LMO2 expression with a moderate and low degree of intensity, respectively.
Conclusions: LMO2 may represent a potentially useful marker to identify T-LBL developing in the context of M/LNs-Eo. In this setting, T-LBL shows LMO2 immunohistochemical profile overlapping with cortical thymocytes and iT-LBP, possibly reflecting different molecular patterns involved in the pathogenesis of T-LBL arising in the setting of M/LNs-Eo.
Keywords: FGFR1; PCM1-JAK2; PDGFRA; PDGFRB; T-cell; eosinophilia; lymphoblastic; lymphoma.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Swerdlow S.H., Campo E., Harris N.L., Jaffe E.S., Pileri S.A., Stein H., Thiele J., editors. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Revised 4th ed. IARC; Lyon, France: 2017.
-
- Pozdnyakova O., Orazi A., Kelemen K., King R., Reichard K.K., E Craig F., Quintanilla-Martinez L., Rimsza L., I George T., Horny H.-P., et al. Myeloid/Lymphoid Neoplasms Associated With Eosinophilia and Rearrangements of PDGFRA, PDGFRB, or FGFR1 or With PCM1-JAK2. Am. J. Clin. Pathol. 2021;155:160–178. doi: 10.1093/ajcp/aqaa208. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

