Predictive Markers of Immunogenicity and Efficacy for Human Vaccines
- PMID: 34205932
- PMCID: PMC8226531
- DOI: 10.3390/vaccines9060579
Predictive Markers of Immunogenicity and Efficacy for Human Vaccines
Abstract
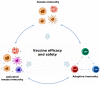
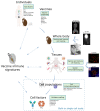
Vaccines represent one of the major advances of modern medicine. Despite the many successes of vaccination, continuous efforts to design new vaccines are needed to fight "old" pandemics, such as tuberculosis and malaria, as well as emerging pathogens, such as Zika virus and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Vaccination aims at reaching sterilizing immunity, however assessing vaccine efficacy is still challenging and underscores the need for a better understanding of immune protective responses. Identifying reliable predictive markers of immunogenicity can help to select and develop promising vaccine candidates during early preclinical studies and can lead to improved, personalized, vaccination strategies. A systems biology approach is increasingly being adopted to address these major challenges using multiple high-dimensional technologies combined with in silico models. Although the goal is to develop predictive models of vaccine efficacy in humans, applying this approach to animal models empowers basic and translational vaccine research. In this review, we provide an overview of vaccine immune signatures in preclinical models, as well as in target human populations. We also discuss high-throughput technologies used to probe vaccine-induced responses, along with data analysis and computational methodologies applied to the predictive modeling of vaccine efficacy.
Keywords: high-throughput technologies; in vivo imaging; machine learning; preclinical models; predictive biomarkers; systems immunology; unsupervised analyses; vaccine signatures; vaccines.
Conflict of interest statement
A.-S.B. is the recipient of Sanofi Innovation Award (iAward program), Europe 2020, on Trained Immunity-Inducing Vaccines. The remaining authors declare no conflict of interest. The funders had no role in the writing of the manuscript.
Figures
References
-
- World Heath Organization (WHO) Global Vaccine Action Plan 2011–2020. [(accessed on 20 May 2021)]; Available online: https://www.who.int/publications/i/item/global-vaccine-action-plan-2011–....
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

