Circular RNAs: Emerging Regulators of the Major Signaling Pathways Involved in Cancer Progression
- PMID: 34205978
- PMCID: PMC8198587
- DOI: 10.3390/cancers13112744
Circular RNAs: Emerging Regulators of the Major Signaling Pathways Involved in Cancer Progression
Abstract
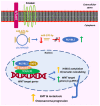
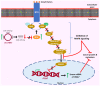
Signal transduction is an essential process that regulates and coordinates fundamental cellular processes, such as development, immunity, energy metabolism, and apoptosis. Through signaling, cells are capable of perceiving their environment and adjusting to changes, and most signaling cascades ultimately lead to alterations in gene expression. Circular RNAs (circRNAs) constitute an emerging type of endogenous transcripts with regulatory roles and unique properties. They are stable and expressed in a tissue-, cell-, and developmental stage-specific manner, while they are involved in the pathogenesis of several diseases, including cancer. Aberrantly expressed circRNAs can mediate cancer progression through regulation of the activity of major signaling cascades, such as the VEGF, WNT/β-catenin, MAPK, PI3K/AKT, and Notch signaling pathways, as well as by interfering with signaling crosstalk. Deregulated signaling can then function to induce angiogenesis, promote invasion, migration, and metastasis, and, generally, modulate the hallmarks of cancer. In this review article, we summarize the most recently described and intriguing cases of circRNA-mediated signaling regulation that are involved in cancer progression, and discuss the biomarker potential of circRNAs, as well as future therapeutic applications.
Keywords: MAPK/ERK; PI3K/AKT; WNT/β-Catenin; biomarkers; circRNA; metastasis; miRNAs; signal transduction; signaling cascades; targeted therapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

