From A to m6A: The Emerging Viral Epitranscriptome
- PMID: 34205979
- PMCID: PMC8227502
- DOI: 10.3390/v13061049
From A to m6A: The Emerging Viral Epitranscriptome
Abstract
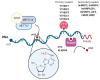
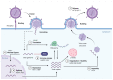
There are over 100 different chemical RNA modifications, collectively known as the epitranscriptome. N6-methyladenosine (m6A) is the most commonly found internal RNA modification in cellular mRNAs where it plays important roles in the regulation of the mRNA structure, stability, translation and nuclear export. This modification is also found in viral RNA genomes and in viral mRNAs derived from both RNA and DNA viruses. A growing body of evidence indicates that m6A modifications play important roles in regulating viral replication by interacting with the cellular m6A machinery. In this review, we will exhaustively detail the current knowledge on m6A modification, with an emphasis on its function in virus biology.
Keywords: RNA modification; epitranscriptomics; m6A; viral infection.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
RNA epitranscriptomics: Regulation of infection of RNA and DNA viruses by N6 -methyladenosine (m6 A).Rev Med Virol. 2018 Jul;28(4):e1983. doi: 10.1002/rmv.1983. Epub 2018 Apr 26. Rev Med Virol. 2018. PMID: 29698584 Free PMC article. Review.
-
The role of N6-methyladenosine (m6A) mRNA modifications in herpesvirus infections.J Virol. 2025 Feb 25;99(2):e0172324. doi: 10.1128/jvi.01723-24. Epub 2025 Jan 27. J Virol. 2025. PMID: 39868828 Free PMC article. Review.
-
N6-methyladenosine (m6A) modification: Emerging regulators in plant-virus interactions.Virology. 2025 Feb;603:110373. doi: 10.1016/j.virol.2024.110373. Epub 2024 Dec 24. Virology. 2025. PMID: 39729962 Review.
-
The RNA Epitranscriptome of DNA Viruses.J Virol. 2018 Oct 29;92(22):e00696-18. doi: 10.1128/JVI.00696-18. Print 2018 Nov 15. J Virol. 2018. PMID: 30185592 Free PMC article. Review.
-
Viral Epitranscriptomics.J Virol. 2017 Apr 13;91(9):e02263-16. doi: 10.1128/JVI.02263-16. Print 2017 May 1. J Virol. 2017. PMID: 28250115 Free PMC article. Review.
Cited by
-
m6A reader proteins: the executive factors in modulating viral replication and host immune response.Front Cell Infect Microbiol. 2023 May 30;13:1151069. doi: 10.3389/fcimb.2023.1151069. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 37325513 Free PMC article. Review.
-
The Epitranscriptome in miRNAs: Crosstalk, Detection, and Function in Cancer.Genes (Basel). 2022 Jul 21;13(7):1289. doi: 10.3390/genes13071289. Genes (Basel). 2022. PMID: 35886072 Free PMC article. Review.
-
Identification and Characterization of BmNPV m6A Sites and Their Possible Roles During Viral Infection.Front Immunol. 2022 Mar 16;13:869313. doi: 10.3389/fimmu.2022.869313. eCollection 2022. Front Immunol. 2022. PMID: 35371067 Free PMC article.
-
Editorial: Viruses and Epitranscriptomes: Regulation of Infection and Antiviral Response.Front Cell Dev Biol. 2022 May 9;10:917894. doi: 10.3389/fcell.2022.917894. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35615700 Free PMC article. No abstract available.
-
Elucidation of the Epitranscriptomic RNA Modification Landscape of Chikungunya Virus.Viruses. 2024 Jun 12;16(6):945. doi: 10.3390/v16060945. Viruses. 2024. PMID: 38932237 Free PMC article.
References
-
- Perry R.P., Kelley D.E. Existence of methylated messenger RNA in mouse L cells. Cell. 1974;1:37–42. doi: 10.1016/0092-8674(74)90153-6. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

