Redox Homeostasis in Muscular Dystrophies
- PMID: 34205993
- PMCID: PMC8229249
- DOI: 10.3390/cells10061364
Redox Homeostasis in Muscular Dystrophies
Abstract
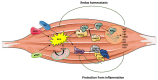
In recent years, growing evidence has suggested a prominent role of oxidative stress in the pathophysiology of several early- and adult-onset muscle disorders, although effective antioxidant treatments are still lacking. Oxidative stress causes cell damage by affecting protein function, membrane structure, lipid metabolism, and DNA integrity, thus interfering with skeletal muscle homeostasis and functionality. Some features related to oxidative stress, such as chronic inflammation, defective regeneration, and mitochondrial damage are shared among most muscular dystrophies, and Nrf2 has been shown to be a central player in antagonizing redox imbalance in several of these disorders. However, the exact mechanisms leading to overproduction of reactive oxygen species and deregulation in the cellular antioxidants system seem to be, to a large extent, disease-specific, and the clarification of these mechanisms in vivo in humans is the cornerstone for the development of targeted antioxidant therapies, which will require testing in appropriately designed clinical trials.
Keywords: FSHD; Nrf2; antioxidants; inflammation; muscular dystrophies; oxidative stress; reactive oxygen species (ROS).
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

