Effects of Dispersants and Biosurfactants on Crude-Oil Biodegradation and Bacterial Community Succession
- PMID: 34206054
- PMCID: PMC8229435
- DOI: 10.3390/microorganisms9061200
Effects of Dispersants and Biosurfactants on Crude-Oil Biodegradation and Bacterial Community Succession
Abstract
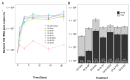
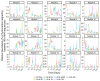
This study evaluated the effects of three commercial dispersants (Finasol OSR 52, Slickgone NS, Superdispersant 25) and three biosurfactants (rhamnolipid, trehalolipid, sophorolipid) in crude-oil seawater microcosms. We analysed the crucial early bacterial response (1 and 3 days). In contrast, most analyses miss this key period and instead focus on later time points after oil and dispersant addition. By focusing on the early stage, we show that dispersants and biosurfactants, which reduce the interfacial surface tension of oil and water, significantly increase the abundance of hydrocarbon-degrading bacteria, and the rate of hydrocarbon biodegradation, within 24 h. A succession of obligate hydrocarbonoclastic bacteria (OHCB), driven by metabolite niche partitioning, is demonstrated. Importantly, this succession has revealed how the OHCB Oleispira, hitherto considered to be a psychrophile, can dominate in the early stages of oil-spill response (1 and 3 days), outcompeting all other OHCB, at the relatively high temperature of 16 °C. Additionally, we demonstrate how some dispersants or biosurfactants can select for specific bacterial genera, especially the biosurfactant rhamnolipid, which appears to provide an advantageous compatibility with Pseudomonas, a genus in which some species synthesize rhamnolipid in the presence of hydrocarbons.
Keywords: OHCB; Oleispira; Pseudomonas; bacteria; biosurfactants; dispersants; hydrocarbons; oil.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Burger J. Oil Spills. Rutgers University Press; New Brunswick, NJ, USA: 1997.
-
- Rystad Energy Global Oil and Gas Discoveries Reach Four-Year High in 2019, Boosted by ExxonMobil’s Guyana Success. [(accessed on 18 May 2020)];2020 Available online: https://www.rystadenergy.com/newsevents/news/press-releases/global-oil-a...
-
- U.S. Energy Information Administration . Short-Term Energy Outlook (STEO) U.S. Energy Information Administration; Washington, DC, USA: 2020.
-
- ITOPF . Use of Dispersants to Treat Oil Spills. Volume 4 ITOPF; London, UK: 2011.
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

