Nanoparticle-Mediated Angiotensin-(1-9) Drug Delivery for the Treatment of Cardiac Hypertrophy
- PMID: 34206106
- PMCID: PMC8228229
- DOI: 10.3390/pharmaceutics13060822
Nanoparticle-Mediated Angiotensin-(1-9) Drug Delivery for the Treatment of Cardiac Hypertrophy
Abstract
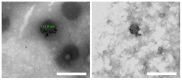
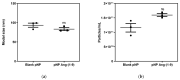
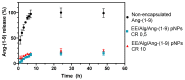
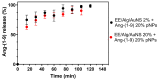
Ang-(1-9) peptide is a bioactive vasodilator peptide that prevents cardiomyocyte hypertrophy in vitro and in vivo as well as lowers blood pressure and pathological cardiovascular remodeling; however, it has a reduced half-life in circulation, requiring a suitable carrier for its delivery. In this work, hybrid nanoparticles composed of polymeric nanoparticles (pNPs) based on Eudragit® E/Alginate (EE/Alg), and gold nanospheres (AuNS), were developed to evaluate their encapsulation capacity and release of Ang-(1-9) under different experimental conditions. Hybrid pNPs were characterized by dynamic light scattering, zeta potential, transmission and scanning electron microscopy, size distribution, and concentration by nanoparticle tracking analysis. Nanometric pNPs, with good polydispersity index and colloidally stable, produced high association efficiency of Ang-(1-9) and controlled release. Finally, the treatment of neonatal cardiomyocytes in culture with EE/Alg/AuNS 2% + Ang-(1-9) 20% pNPs decreased the area and perimeter, demonstrating efficacy in preventing norepinephrine-induced cardiomyocyte hypertrophy. On the other hand, the incorporation of AuNS did not cause negative effects either on the cytotoxicity or on the association capacity of Ang-(1-9), suggesting that the hybrid carrier EE/Alg/AuNS pNPs could be used for the delivery of Ang-(1-9) in the treatment of cardiovascular hypertrophy.
Keywords: angiotensin-(1-9); cardiac hypertrophy; gold nanoparticles; peptide delivery; polymeric nanoparticles.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- World Health Organization Cardiovascular Diseases. [(accessed on 25 January 2021)]; Available online: https://www.who.int/nmh/publications/fact_sheet_cardiovascular_en.pdf.
Grants and funding
- FONDECYT Regular 1181689/Agencia Nacional de Investigación y Desarrollo
- PIA ACT192144/Agencia Nacional de Investigación y Desarrollo
- FONDAP 15130011/Agencia Nacional de Investigación y Desarrollo
- National Doctoral Scholarship N°21130766/Agencia Nacional de Investigación y Desarrollo
- National Doctoral Scholarship N°21141219/Agencia Nacional de Investigación y Desarrollo
LinkOut - more resources
Full Text Sources
Miscellaneous

