Caprine Arthritis Encephalitis Virus Is Associated with Renal Lesions
- PMID: 34206110
- PMCID: PMC8230173
- DOI: 10.3390/v13061051
Caprine Arthritis Encephalitis Virus Is Associated with Renal Lesions
Abstract
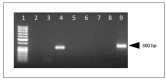
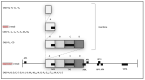
Caprine arthritis encephalitis virus (CAEV) is a monocyte/macrophage-tropic lentivirus that primarily infects goats resulting in a well-recognized set of chronic inflammatory syndromes focused on the joint synovium, tissues of the central nervous system, pulmonary interstitium and mammary gland. Clinically affected animals generally manifest with one or more of these classic CAEV-associated tissue lesions; however, CAEV-associated renal inflammation in goats has not been reported in the peer-reviewed literature. Here we describe six goats with chronic, multisystemic CAEV infections in conjunction with CAEV-associated renal lesions. One of the animals had CAEV antigen-associated thrombotic arteritis resulting in infarction of both the kidney and heart. These goats had microscopic evidence of inflammatory renal injury (interstitial nephritis) with detectable renal immunolabeling for CAEV antigen in three of six animals and amplifiable proviral sequences consistent with CAEV in all six animals. Cardiac lesions (vascular, myocardial or endocardial) were also identified in four of six animals. Within the viral promoter (U3) region, known transcription factor binding sites (TFBSs) were generally conserved, although one viral isolate had a duplication of the U3 A region encoding a second gamma-activated site (GAS). Despite the TFBS conservation, the isolates demonstrated a degree of phylogenetic diversity. At present, the clinical consequence of CAEV-associated renal injury is not clear.
Keywords: CAEV; SRLV; TFBS; goat; interstitial nephritis; kidney; lentivirus; promoter.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Fields B.N., Knipe D.M., Howley P.M., Griffin D.E. Fields Virology. 4th ed. Lippincott Williams & Wilkins; Philadelphia, PA, USA: 2001. p. 3087.
-
- Crawford T.B., Adams D.S. Caprine arthritis-encephalitis: Clinical features and presence of antibody in selected goat populations. J. Am. Vet. Med. Assoc. 1981;178:713–719. - PubMed

