Role of Receptor Protein Tyrosine Phosphatase β/ζ in Neuron-Microglia Communication in a Cellular Model of Parkinson's Disease
- PMID: 34206170
- PMCID: PMC8269034
- DOI: 10.3390/ijms22136646
Role of Receptor Protein Tyrosine Phosphatase β/ζ in Neuron-Microglia Communication in a Cellular Model of Parkinson's Disease
Abstract
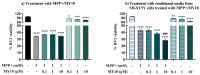
Pleiotrophin (PTN) is a neurotrophic factor that regulates glial responses in animal models of different types of central nervous system (CNS) injuries. PTN is upregulated in the brain in different pathologies characterized by exacerbated neuroinflammation, including Parkinson's disease. PTN is an endogenous inhibitor of Receptor Protein Tyrosine Phosphatase (RPTP) β/ζ, which is abundantly expressed in the CNS. Using a specific inhibitor of RPTPβ/ζ (MY10), we aimed to assess whether the PTN/RPTPβ/ζ axis is involved in neuronal and glial injury induced by the toxin MPP+. Treatment with the RPTPβ/ζ inhibitor MY10 alone decreased the viability of both SH-SY5Y neuroblastoma cells and BV2 microglial cultures, suggesting that normal RPTPβ/ζ function is involved in neuronal and microglial viability. We observed that PTN partially decreased the cytotoxicity induced by MPP+ in SH-SY5Y cells underpinning the neuroprotective function of PTN. However, MY10 did not seem to modulate the SH-SY5Y cell loss induced by MPP+. Interestingly, we observed that media from SH-SY5Y cells treated with MPP+ and MY10 decreases microglial viability but may elicit a neuroprotective response of microglia by upregulating Ptn expression. The data suggest a neurotrophic role of microglia in response to neuronal injury through upregulation of Ptn levels.
Keywords: MPP+; RPTP β/ζ; microglia; midkine; neuroinflammation; pleiotrophin.
Conflict of interest statement
The authors declare that there are no conflict of interest with respect to the conducted research, authorship, and publication of this work.
Figures




Similar articles
-
Role of RPTPβ/ζ in neuroinflammation and microglia-neuron communication.Sci Rep. 2020 Nov 20;10(1):20259. doi: 10.1038/s41598-020-76415-5. Sci Rep. 2020. PMID: 33219280 Free PMC article.
-
Inhibition of RPTPβ/ζ blocks ethanol-induced conditioned place preference in pleiotrophin knockout mice.Behav Brain Res. 2019 Sep 2;369:111933. doi: 10.1016/j.bbr.2019.111933. Epub 2019 May 1. Behav Brain Res. 2019. PMID: 31054277
-
Anaplastic lymphoma kinase: "Ligand Independent Activation" mediated by the PTN/RPTPβ/ζ signaling pathway.Biochim Biophys Acta. 2013 Oct;1834(10):2219-23. doi: 10.1016/j.bbapap.2013.06.004. Epub 2013 Jun 15. Biochim Biophys Acta. 2013. PMID: 23777859 Review.
-
Pharmacological inhibition of Receptor Protein Tyrosine Phosphatase β/ζ (PTPRZ1) modulates behavioral responses to ethanol.Neuropharmacology. 2018 Jul 15;137:86-95. doi: 10.1016/j.neuropharm.2018.04.027. Epub 2018 May 9. Neuropharmacology. 2018. PMID: 29753117 Free PMC article.
-
Pleiotrophin and its receptor protein tyrosine phosphatase beta/zeta as regulators of angiogenesis and cancer.Biochim Biophys Acta. 2016 Dec;1866(2):252-265. doi: 10.1016/j.bbcan.2016.09.007. Epub 2016 Sep 29. Biochim Biophys Acta. 2016. PMID: 27693125 Review.
Cited by
-
Pharmacological inhibition of receptor protein tyrosine phosphatase β/ζ decreases Aβ plaques and neuroinflammation in the hippocampus of APP/PS1 mice.Front Pharmacol. 2024 Dec 6;15:1506049. doi: 10.3389/fphar.2024.1506049. eCollection 2024. Front Pharmacol. 2024. PMID: 39712494 Free PMC article.
-
Protein Tyrosine Phosphatases in Neuroblastoma: Emerging Roles as Biomarkers and Therapeutic Targets.Front Cell Dev Biol. 2021 Dec 8;9:811297. doi: 10.3389/fcell.2021.811297. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34957126 Free PMC article. Review.
-
Inhibition of RPTPβ/ζ reduces chronic ethanol intake in adolescent mice and modulates ethanol effects on hippocampal neurogenesis and glial responses in a sex-dependent manner.Neuropharmacology. 2023 Apr 1;227:109438. doi: 10.1016/j.neuropharm.2023.109438. Epub 2023 Jan 24. Neuropharmacology. 2023. PMID: 36706907 Free PMC article.
-
Pleiotrophin and the Expression of Its Receptors during Development of the Human Cerebellar Cortex.Cells. 2023 Jun 27;12(13):1733. doi: 10.3390/cells12131733. Cells. 2023. PMID: 37443767 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

