Osteoblast Differentiation and Signaling: Established Concepts and Emerging Topics
- PMID: 34206294
- PMCID: PMC8268587
- DOI: 10.3390/ijms22136651
Osteoblast Differentiation and Signaling: Established Concepts and Emerging Topics
Abstract
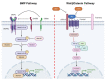
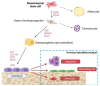
Osteoblasts, the cells that build up our skeleton, are remarkably versatile and important cells that need tight regulation in all the phases of their differentiation to guarantee proper skeletal development and homeostasis. Although we know many of the key pathways involved in osteoblast differentiation and signaling, it is becoming clearer and clearer that this is just the tip of the iceberg, and we are constantly discovering novel concepts in osteoblast physiology. In this review, we discuss well-established pathways of osteoblastic differentiation, i.e., the classical ones committing mesenchymal stromal cells to osteoblast, and then osteocytes as well as recently emerged players. In particular, we discuss micro (mi)RNAs, long non-coding (lnc)RNAs, circular (circ)RNAs, and extracellular vesicles, focusing on the mechanisms through which osteoblasts are regulated by these factors, and conversely, how they use extracellular vesicles to communicate with the surrounding microenvironment.
Keywords: extracellular vesicles; miRNAs; non-coding RNAs; osteoblast differentiation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Raisz L.G. Physiology and Pathophysiology of Bone Remodeling. Proc. Clin. Chem. 1999;45:1353–1358. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

