Nuclear Export Inhibitor KPT-8602 Synergizes with PARP Inhibitors in Escalating Apoptosis in Castration Resistant Cancer Cells
- PMID: 34206543
- PMCID: PMC8268282
- DOI: 10.3390/ijms22136676
Nuclear Export Inhibitor KPT-8602 Synergizes with PARP Inhibitors in Escalating Apoptosis in Castration Resistant Cancer Cells
Abstract
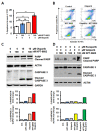
Aberrant nuclear protein transport, often observed in cancer, causes mislocalization-dependent inactivation of critical cellular proteins. Earlier we showed that overexpression of exportin 1 is linked to higher grade and Gleason score in metastatic castration resistant prostate cancer (mCRPC). We also showed that a selective inhibitor of nuclear export (SINE) selinexor and second generation eltanexor (KPT-8602) could suppress mCRPC growth, reduce androgen receptor (AR), and re-sensitize to androgen deprivation therapy. Here we evaluated the combination of KPT-8602 with PARP inhibitors (PARPi) olaparib, veliparib and rucaparib in 22rv1 mCRPC cells. KPT-8602 synergized with PARPi (CI < 1) at pharmacologically relevant concentrations. KPT-8602-PARPi showed superior induction of apoptosis compared to single agent treatment and caused up-regulation of pro-apoptotic genes BAX, TP53 and CASPASE 9. Mechanistically, KPT-8602-PARPi suppressed AR, ARv7, PSA and AR targets FOXA1 and UBE2C. Western blot analysis revealed significant down-regulation of AR, ARv7, UBE2C, SAM68, FOXA1 and upregulation of cleaved PARP and cleaved CASPASE 3. KPT-8602 with or without olaparib was shown to reduce homologous recombination-regulated DNA damage response targets including BRCA1, BRCA2, CHEK1, EXO1, BLM, RAD51, LIG1, XRCC3 and RMI2. Taken together, this study revealed the therapeutic potential of a novel combination of KPT-8602 and PARP inhibitors for the treatment of mCRPC.
Keywords: KPT-8602; PARP inhibitors; castration resistance; eltanexor; nuclear export; prostate cancer.
Conflict of interest statement
Direct Conflict of Interest: Yosef Landesman and Trinayan Kashyap are employees at Karyopharm Therapeutics Inc. Asfar S. Azmi received speaker fees from Karyopharm. Unrelated Conflict Disclosure: Asfar S. Azmi received funding from Rhizen Pharmaceutics Inc. EISAI and Janssen. Md. Hafiz Uddin, Yiwei Li, Husain Yar Khan, Irfana Muqbil, Amro Aboukameel, Rachel E. Sexton, Shriya Reddy, and Elisabeth I. Heath declare no conflict of interest.
Figures




Similar articles
-
Clinical Development of PARP Inhibitors in Treating Metastatic Castration-Resistant Prostate Cancer.Cells. 2019 Aug 9;8(8):860. doi: 10.3390/cells8080860. Cells. 2019. PMID: 31404966 Free PMC article. Review.
-
The emerging role of PARP inhibitors in prostate cancer.Expert Rev Anticancer Ther. 2020 Aug;20(8):715-726. doi: 10.1080/14737140.2020.1797497. Epub 2020 Aug 6. Expert Rev Anticancer Ther. 2020. PMID: 32758032 Review.
-
Androgen receptor inhibitor-induced "BRCAness" and PARP inhibition are synthetically lethal for castration-resistant prostate cancer.Sci Signal. 2017 May 23;10(480):eaam7479. doi: 10.1126/scisignal.aam7479. Sci Signal. 2017. PMID: 28536297 Free PMC article.
-
Attenuation of SRC Kinase Activity Augments PARP Inhibitor-mediated Synthetic Lethality in BRCA2-altered Prostate Tumors.Clin Cancer Res. 2021 Mar 15;27(6):1792-1806. doi: 10.1158/1078-0432.CCR-20-2483. Epub 2020 Dec 17. Clin Cancer Res. 2021. PMID: 33334906 Free PMC article.
-
Differential Activity of PARP Inhibitors in BRCA1- Versus BRCA2-Altered Metastatic Castration-Resistant Prostate Cancer.JCO Precis Oncol. 2021 Jul 22;5:PO.21.00070. doi: 10.1200/PO.21.00070. eCollection 2021. JCO Precis Oncol. 2021. PMID: 34778690 Free PMC article.
Cited by
-
Alteration in expression and subcellular localization of the androgen receptor- regulated FAM111A protease is associated with emergence of castration resistant prostate cancer.Neoplasia. 2025 Aug;66:101181. doi: 10.1016/j.neo.2025.101181. Epub 2025 May 29. Neoplasia. 2025. PMID: 40446667 Free PMC article.
-
Role of Sam68 in different types of cancer (Review).Int J Mol Med. 2025 Jan;55(1):3. doi: 10.3892/ijmm.2024.5444. Epub 2024 Oct 25. Int J Mol Med. 2025. PMID: 39450529 Free PMC article. Review.
-
Therapeutic Targeting of Exportin-1 in Childhood Cancer.Cancers (Basel). 2021 Dec 7;13(24):6161. doi: 10.3390/cancers13246161. Cancers (Basel). 2021. PMID: 34944778 Free PMC article. Review.
-
The nuclear export protein exportin-1 in solid malignant tumours: From biology to clinical trials.Clin Transl Med. 2024 May;14(5):e1684. doi: 10.1002/ctm2.1684. Clin Transl Med. 2024. PMID: 38783482 Free PMC article. Review.
-
CRM1 regulates androgen receptor stability and impacts DNA repair pathways in prostate cancer, independent of the androgen receptor.FASEB J. 2025 Jan 31;39(2):e70342. doi: 10.1096/fj.202400490R. FASEB J. 2025. PMID: 39873970 Free PMC article.
References
-
- Donovan M.J., Osman I., Khan F.M., Vengrenyuk Y., Capodieci P., Koscuiszka M., Anand A., Cordon-Cardo C., Costa J., Scher H.I. Androgen receptor expression is associated with prostate cancer-specific survival in castrate patients with metastatic disease. BJU Int. 2010;105:462–467. doi: 10.1111/j.1464-410X.2009.08747.x. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

