Novel Bradykinin Receptor Inhibitors Inhibit Proliferation and Promote the Apoptosis of Hepatocellular Carcinoma Cells by Inhibiting the ERK Pathway
- PMID: 34206871
- PMCID: PMC8272207
- DOI: 10.3390/molecules26133915
Novel Bradykinin Receptor Inhibitors Inhibit Proliferation and Promote the Apoptosis of Hepatocellular Carcinoma Cells by Inhibiting the ERK Pathway
Abstract
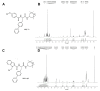
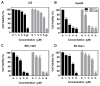
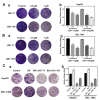
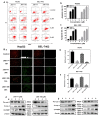
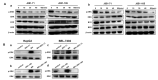
Hepatocellular carcinoma (HCC) is the fifth most common cancer worldwide. Studies have shown that bradykinin (BK) is highly expressed in liver cancer. We designed the novel BK receptor inhibitors J051-71 and J051-105, which reduced the viability of liver cancer cells and inhibited the formation of cancer cell colonies. J051-71 and J051-105 reduced cell proliferation and induced apoptosis in HepG2 and BEL-7402 cells, which may be due to the inhibition of the extracellular regulated protein kinase (ERK) signaling pathway. In addition, these BK receptor inhibitors reversed the cell proliferation induced by BK in HepG2 and BEL-7402 cells by downregulating B1 receptor expression. Inhibiting B1 receptor expression decreased the protein levels of p-ERK and reduced the malignant progression of HCC, providing a potential target for HCC therapy.
Keywords: ERK signaling pathway; apoptosis; bradykinin B1 receptor; bradykinin receptor inhibitor; hepatocellular carcinoma.
Conflict of interest statement
The authors declare no conflict of interest with the contents of this article.
Figures
References
-
- Yang Y., Nagano H., Ota H., Morimoto O., Nakamura M., Wada H., Node T., Damdinsuren B., Marubashi S., Miyamoto A., et al. Patterns and clinicopathologic features of extrahepatic recurrence of hepatocellular carcinoma after curative resection. Surgery. 2007;141:196–202. doi: 10.1016/j.surg.2006.06.033. - DOI - PubMed
-
- Kudo M., Finn R.S., Qin S., Han K.H., Ikeda K., Piscaglia F., Baron A., Park J.-W., Han G., Jassem J., et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163–1173. doi: 10.1016/S0140-6736(18)30207-1. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

