How to Obtain a High Quality ctDNA in Lymphoma Patients: Preanalytical Tips and Tricks
- PMID: 34206947
- PMCID: PMC8308879
- DOI: 10.3390/ph14070617
How to Obtain a High Quality ctDNA in Lymphoma Patients: Preanalytical Tips and Tricks
Abstract
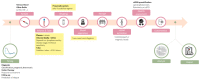
The analysis of circulating tumor DNA (ctDNA) released by tumor cells holds great promise for patients with lymphoma, to refine the diagnostic procedure, clarify the prognosis, monitor the response to treatment, and detect relapses earlier. One of the main challenges of the coming years is to adapt techniques from highly specialized translational teams to routine laboratories as this requires a careful technical and clinical validation, and we have to achieve this as fast as possible to transform a promising biomarker into a routine analysis to have a direct consequence on patient care. Whatever the analytical technology used, the prerequisite is to obtain high yields of ctDNA of optimal quality. In this review, we propose a step-by-step description of the preanalytical process to obtain high-quality ctDNA, emphasizing the technical choices that need to be made and the experimental data that can support these choices.
Keywords: ctDNA; lymphoma; preanalytical.
Conflict of interest statement
P.S. and S.H. are members of the OngNGS consortium. The other authors declare no conflict of interest.
Figures
References
-
- Mandel P., Metais P. Nuclear Acids In Human Blood Plasma. C. R. Seances Soc. Biol. Fil. 1948;142:241–243. - PubMed
-
- Jahr S., Hentze H., Englisch S., Hardt D., Fackelmayer F.O., Hesch R.-D., Knippers R. DNA Fragments in the Blood Plasma of Cancer Patients: Quantitations and Evidence for Their Origin from Apoptotic and Necrotic Cells. Cancer Res. 2001;61:1659–1665. - PubMed
Publication types
LinkOut - more resources
Full Text Sources