Characterization of βN-Octadecanoyl-5-hydroxytryptamide Anti-Inflammatory Effect
- PMID: 34206998
- PMCID: PMC8234578
- DOI: 10.3390/molecules26123709
Characterization of βN-Octadecanoyl-5-hydroxytryptamide Anti-Inflammatory Effect
Abstract
Background: N-octadecanoyl-5-hydroxytryptamide (C18-5HT) is an amide that can be obtained by the coupling of serotonin and octadecanoic acid. This study aims to characterize the in vivo and in vitro anti-inflammatory activity of C18-5HT.
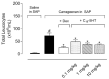
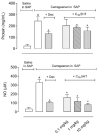
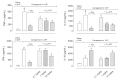
Methods: A subcutaneous air pouch model (SAP) was used. The exudates were collected from SAP after carrageenan injection to assess cell migration and inflammatory mediators production. RAW 264.7 cells were used for in vitro assays.
Results: C18-5HT significantly inhibited leukocyte migration into the SAP as well as nitric oxide (NO) and cytokines production and protein extravasation. We also observed an reduction in some cytokines and an increase in IL-10 production. Assays conducted with RAW 264.7 cells indicated that C18-5HT inhibited NO and cytokine produced.
Conclusions: Taken together, our data suggest that C18-5HT presents a significant effect in different cell types (leukocytes collected from exudate, mainly polumorphonuclear leukocytes and cell culture macrophages) and is a promising compound for further studies for the development of a new anti-inflammatory drug.
Keywords: C18-5HT; N-octadecanoyl-5-hydroxytryptamide; anti-inflammatory activity; inflammation; serotonin amides.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

