Allergen Exposure in Murine Neonates Promoted the Development of Asthmatic Lungs
- PMID: 34207237
- PMCID: PMC8235458
- DOI: 10.3390/biomedicines9060688
Allergen Exposure in Murine Neonates Promoted the Development of Asthmatic Lungs
Abstract
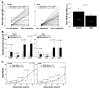
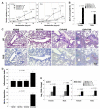
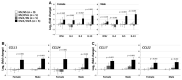
We previously demonstrated that fetal allergen exposure caused T-helper 2 (Th2) cell sensitization. Although neonates are immunologically more mature than fetuses, asthmatic lungs were reportedly mitigated by neonatal allergen administration, mechanically referring to regulatory T-cells and TGF-β signaling but lacking the immunological profiles after neonatal exposure. To reappraise the immunological outcome of neonatal allergen exposure, we injected adjuvant-free ovalbumin intraperitoneally into 2-day-old BALB/c neonates, followed by aerosolized ovalbumin inhalation in adulthood. Mice were examined for the immunological profiles specifically after neonatal exposures, lung function and histology (hematoxylin-eosin or periodic acid Schiff staining), and gene expressions of intrapulmonary cytokines (IL-4, IL-5, IL-13 and IFN-γ) and chemokines (CCL17, CCL22, CCL11 and CCL24). Neonatal ovalbumin exposure triggered Th2-skewed sensitization and ovalbumin-specific IgE production. Subsequent ovalbumin inhalation in adulthood boosted Th2 immunity and caused asthmatic lungs with structural and functional alterations of airways. Gender difference mainly involved airway hyperresponsiveness and resistance with greater female susceptibility to methacholine bronchospastic stimulation. In lungs, heightened chemoattractant gene expressions were only granted to neonatally ovalbumin-sensitized mice with aerosolized ovalbumin stress in adulthood, and paralleled by upregulated Th2 cytokine genes. Thus, aeroallergen stress in atopic individuals might upregulate the expression of intrapulmonary chemoattractants to recruit Th2 cells and eosinophils into the lungs, pathogenically linked to asthma development. Conclusively, murine neonates were sensitive to allergen exposures. Exposure events during neonatal stages were crucial to asthma predisposition in later life. These findings from a murine model point to allergen avoidance in neonatal life, possibly even very early in utero, as the best prospect of primary asthma prevention.
Keywords: adjuvant; asthma; chemokine; cytokine; neonate; ovalbumin; sensitization.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures



References
-
- Chen J.-C., Chan C.-C., Wu C.-J., Ou L.-S., Yu H.-Y., Chang H.-L., Tseng L.-Y., Kuo M.-L. Fetal Phagocytes Take up Allergens to Initiate T-Helper Cell Type 2 Immunity and Facilitate Allergic Airway Responses. Am. J. Respir. Crit. Care Med. 2016;194:934–947. doi: 10.1164/rccm.201508-1703OC. - DOI - PubMed
-
- Chen J.-C., Kuo M.-L., Ou L.-S., Chang P.-Y., Muench M.O., Shen C.-R., Chang H.-L., Yu H.-Y., Fu R.-H. Characterization of Tolerance Induction through Prenatal Marrow Transplantation: The Requirement for a Threshold Level of Chimerism to Establish Rather than Maintain Postnatal Skin Tolerance. Cell Transplant. 2010;19:1609–1622. doi: 10.3727/096368910X516583. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

