CRISPR-Associated (CAS) Effectors Delivery via Microfluidic Cell-Deformation Chip
- PMID: 34207502
- PMCID: PMC8226447
- DOI: 10.3390/ma14123164
CRISPR-Associated (CAS) Effectors Delivery via Microfluidic Cell-Deformation Chip
Abstract
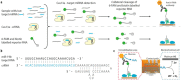
Identifying new and even more precise technologies for modifying and manipulating selectively specific genes has provided a powerful tool for characterizing gene functions in basic research and potential therapeutics for genome regulation. The rapid development of nuclease-based techniques such as CRISPR/Cas systems has revolutionized new genome engineering and medicine possibilities. Additionally, the appropriate delivery procedures regarding CRISPR/Cas systems are critical, and a large number of previous reviews have focused on the CRISPR/Cas9-12 and 13 delivery methods. Still, despite all efforts, the in vivo delivery of the CAS gene systems remains challenging. The transfection of CRISPR components can often be inefficient when applying conventional delivery tools including viral elements and chemical vectors because of the restricted packaging size and incompetency of some cell types. Therefore, physical methods such as microfluidic systems are more applicable for in vitro delivery. This review focuses on the recent advancements of microfluidic systems to deliver CRISPR/Cas systems in clinical and therapy investigations.
Keywords: CRISPR; Cas9 protein; genome; microfluidics; tissue engineering.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Challenges in CRISPR/CAS9 Delivery: Potential Roles of Nonviral Vectors.Hum Gene Ther. 2015 Jul;26(7):452-62. doi: 10.1089/hum.2015.069. Hum Gene Ther. 2015. PMID: 26176432 Review.
-
Gene Therapy with CRISPR/Cas9 Coming to Age for HIV Cure.AIDS Rev. 2017 Oct-Dec;19(3):167-172. AIDS Rev. 2017. PMID: 29019352
-
CRISPR/Cas9-mediated genome editing: From basic research to translational medicine.J Cell Mol Med. 2020 Apr;24(7):3766-3778. doi: 10.1111/jcmm.14916. Epub 2020 Feb 25. J Cell Mol Med. 2020. PMID: 32096600 Free PMC article. Review.
-
Progress of delivery methods for CRISPR-Cas9.Expert Opin Drug Deliv. 2022 Aug;19(8):913-926. doi: 10.1080/17425247.2022.2100342. Epub 2022 Jul 17. Expert Opin Drug Deliv. 2022. PMID: 35818792 Review.
-
Delivery of CRISPR/Cas9 for therapeutic genome editing.J Gene Med. 2019 Jul;21(7):e3107. doi: 10.1002/jgm.3107. J Gene Med. 2019. PMID: 31237055 Review.
Cited by
-
Recent Advances in CRISPR/Cas9 Delivery Approaches for Therapeutic Gene Editing of Stem Cells.Stem Cell Rev Rep. 2023 Nov;19(8):2576-2596. doi: 10.1007/s12015-023-10585-3. Epub 2023 Sep 18. Stem Cell Rev Rep. 2023. PMID: 37723364 Free PMC article. Review.
-
Utilization of CRISPR-Mediated Tools for Studying Functional Genomics in Hematological Malignancies: An Overview on the Current Perspectives, Challenges, and Clinical Implications.Front Genet. 2022 Jan 28;12:767298. doi: 10.3389/fgene.2021.767298. eCollection 2021. Front Genet. 2022. PMID: 35154242 Free PMC article. Review.
-
Exploring the Role of the TGF-β Signaling Pathway in Colorectal Precancerous Polyps Biochemical Genetics.Biochem Genet. 2025 Apr;63(2):1116-1148. doi: 10.1007/s10528-024-10988-y. Epub 2024 Dec 5. Biochem Genet. 2025. PMID: 39636332 Review.
-
Stem cell therapy for COVID-19 pneumonia.Mol Biomed. 2022 Feb 17;3(1):6. doi: 10.1186/s43556-021-00067-8. Mol Biomed. 2022. PMID: 35174448 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources

